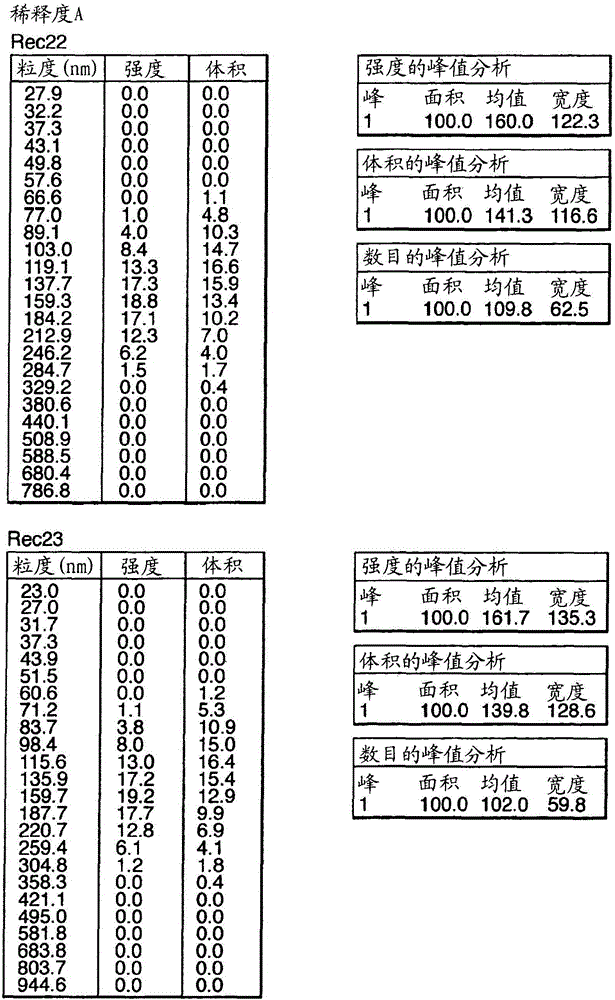
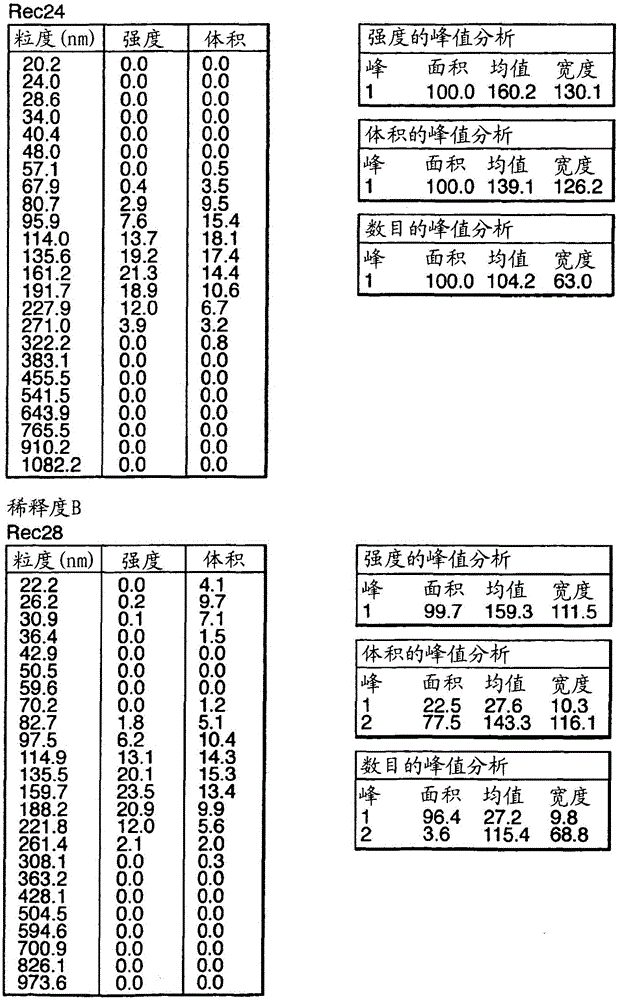
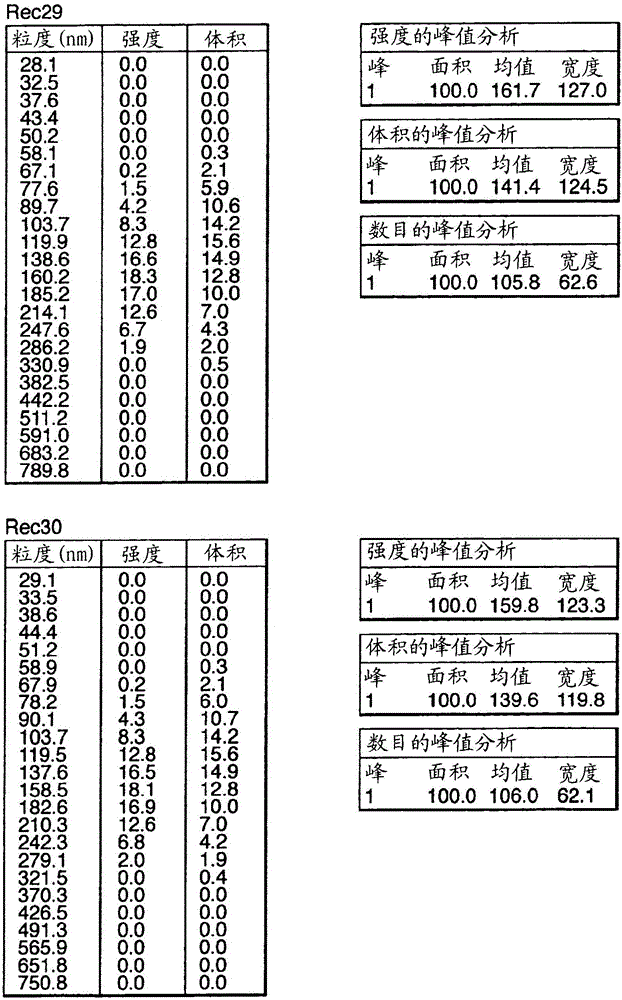
Influenza vaccine
An influenza vaccine, influenza virus technology, applied in the field of preparation, monovalent influenza immunogenic composition, increasing the immune response to various antigens, can solve the problem of no pattern and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0221] Example 1 - Immune readout method
[0222] I.1. Ferret method
[0223] A suitable method is as follows, which is usually used for experiments with seasonal strains. The skilled reader will understand that some modifications or optimizations may be made depending on the strain of influenza virus used.
[0224] I.1.1. Hemagglutination inhibition test (HI)
[0225] test program
[0226] Anti-hemagglutinin antibody titers against influenza virus strains were determined using the hemagglutination inhibition test (HI). The principle of the HI test is based on the ability of specific anti-influenza antibodies to inhibit the hemagglutination of equine red blood cells (RBC) by influenza virus hemagglutinin (HA). After pretreatment of sera (cholera, RDE, heat inactivated), two-fold dilutions of sera were incubated with 4 hemagglutination units of the influenza virus strain. Equine (adapt: turkey or horse) erythrocytes were then added and inhibition of agglutination was r...
Embodiment II
[0303] Example II - Preparation and Characterization of Oil-in-Water Emulsion and Adjuvant Formulations
[0304] Unless otherwise stated, the oil / water emulsions used in the subsequent examples consisted of an organic phase consisting of two oils (α-tocopherol and squalene) and a PBS aqueous phase containing Tween 80 as an emulsifier. Unless otherwise stated, the oil-in-water emulsion adjuvant formulations used in the subsequent examples were made to contain the following oil-in-water emulsion components (final concentrations given): 2.5% squalene (v / v), 2.5% alpha-tocopherol (v / v), 0.9% polyoxyethylene sorbitan monooleate (v / v) (Tween80), see WO95 / 17210. This emulsion, referred to as AS03 in subsequent examples, was prepared as a 2-fold concentrate as follows.
[0305] II.1. Preparation of emulsion SB62
[0306] II.1.1. Laboratory scale preparation
[0307] Tween80 was dissolved in phosphate buffered saline (PBS) to obtain a 2% PBS solution. To provide 100ml of 2x conce...
Embodiment III
[0354] Example III - Preclinical Evaluation of Adjuvanted Pandemic Split Influenza Vaccines (Containing H5N1 Strains) in Ferrets
[0355] III.1. Rationale and objectives
[0356] Considering infection susceptibility and clinical response, ferret-mode influenza infection closely resembles human influenza. Ferrets are highly susceptible to infection with influenza A and B viruses (excluding previously employed strains). Therefore, it provides an excellent model system for the study of protection conferred by administration of influenza vaccines.
[0357] This study investigated the protective effect of AS03-adjuvanted H5N1 split vaccine against ferrets challenged with H5N1 homostrain A / Vietnam / 1194 / 2004 or with allogeneic A / Indonesia. The goal of the experiment was to demonstrate the effect of adjuvanted influenza vaccine compared to ferrets immunized with PBS or adjuvant alone.
[0358] III.2. Experimental design
[0359] III.2.1. Treatment / Group (Table 4)
[0360] On da...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com