In-vitro recombined human skin epidermis model and preparation method and application thereof
A human skin and external recombination technology, applied in biochemical equipment and methods, epidermal cells/skin cells, tissue screening, etc., can solve problems such as model shrinkage, link structure defects, leakage, etc., to improve the screening rate, The effect of increasing the surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
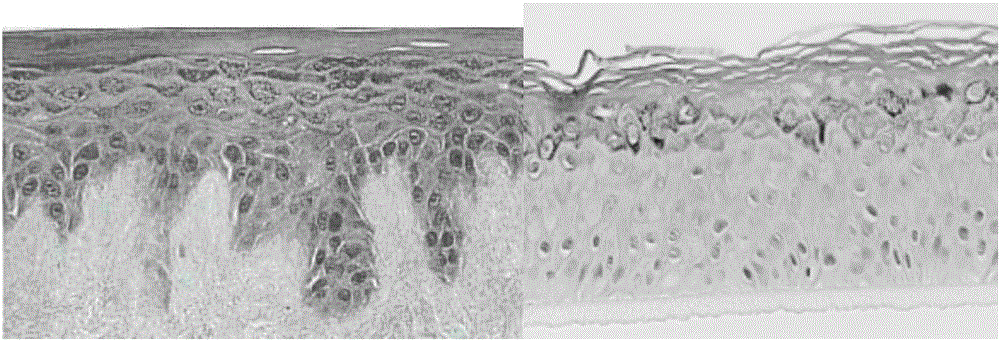
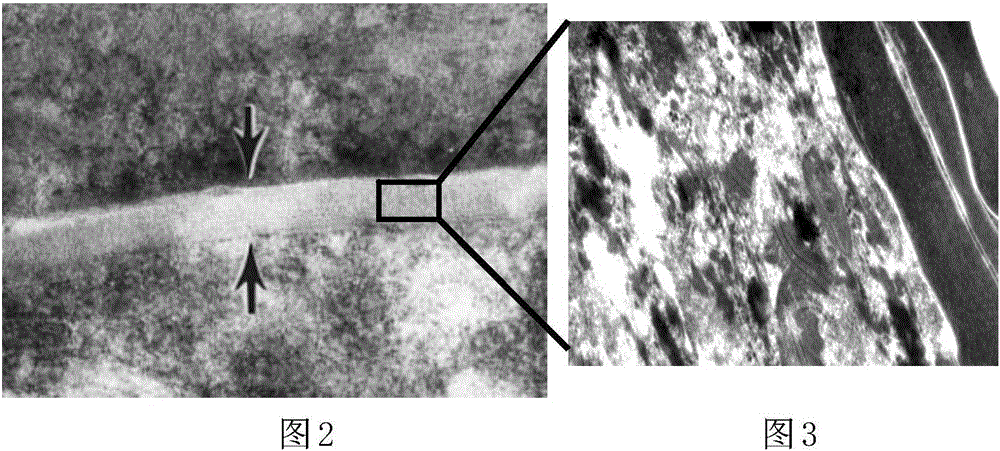
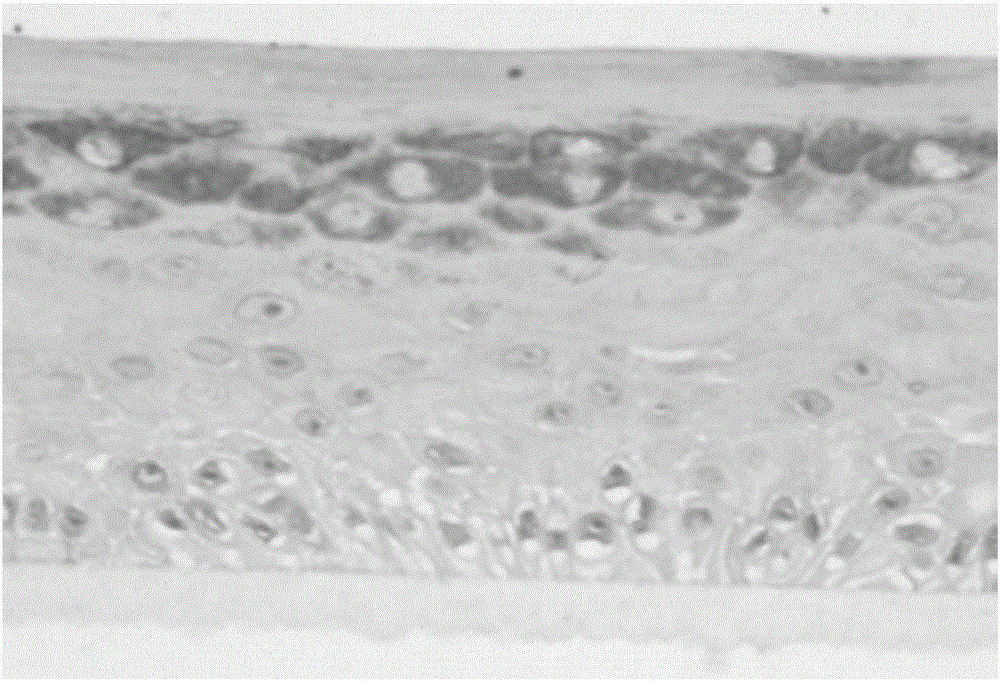
[0102] The in vitro recombinant human skin epidermis model in this implementation is composed of basal cell layer, spinous cell layer, granule cell layer and keratinocyte layer. The lower surface of the basal cell layer has a layer of hemidesmosomal protein, and the upper surface of the basal cell layer is covered with a spinous cell layer, which is composed of 7 layers of spiny cells with short protrusions, and the upper surface of the spinous cell layer The surface is covered with a layer of granule cells, which consists of three layers of cells containing transparent granules, and the upper surface of the layer of granule cells is covered with a layer of corneocytes. The thickness of the in vitro recombinant human skin epidermis model in this embodiment is 120 μm.
[0103] The preparation method of the above-mentioned in vitro recombinant human skin epidermis model consists of the following steps:
[0104] 1. Preparation of Matrigel Microparticles
[0105] Dilute Matrigel...
Embodiment 2
[0124] The in vitro recombinant human skin epidermis model in this implementation is composed of basal cell layer, spinous cell layer, granule cell layer and keratinocyte layer. The lower surface of the basal cell layer has a layer of hemidesmosomal protein, and the upper surface of the basal cell layer is covered with a spinous cell layer, which is composed of 6 layers of spiny cells with short protrusions, and the upper surface of the spinous cell layer The surface is covered with a layer of granule cells, which consists of three layers of cells containing transparent granules, and the upper surface of the layer of granule cells is covered with a layer of corneocytes. The thickness of the in vitro recombinant human skin epidermis model in this embodiment is 120 μm.
[0125] Its preparation method is as follows:
[0126] 1. Preparation of Matrigel Microparticles
[0127] This step is the same as in Example 1.
[0128] 2. Screen and expand epidermal stem cells
[0129] Imm...
Embodiment 3
[0142] The in vitro recombinant human skin epidermis model in this implementation is composed of basal cell layer, spinous cell layer, granule cell layer and keratinocyte layer. The lower surface of the basal cell layer has a layer of hemidesmosomal protein, and the upper surface of the basal cell layer is covered with a spinous cell layer, which is composed of 8 layers of spiny cells with short protrusions, and the upper surface of the spinous cell layer The surface is covered with a granule cell layer, which is composed of 4 layers of cells containing transparent granules, and the upper surface of the granule cell layer is covered with a keratinocyte layer. The thickness of the in vitro recombinant human skin epidermis model in this embodiment is 150 μm.
[0143] Its preparation method is as follows:
[0144] 1. Preparation of Matrigel Microparticles
[0145] This step is the same as in Example 1.
[0146] 2. Screen and expand epidermal stem cells
[0147] Immerse the is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com