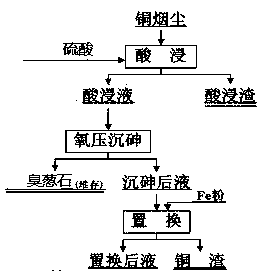
A method for recovering copper and arsenic from copper fumes and solidifying them into scorodite
A technology for scorodite and copper recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 1). Acid leaching the copper fumes to obtain an acid leaching solution. Take 700g of copper fume (Fe is 2~12%, As is 4~12%, Cu is 3~6%, Zn is 7~10%, Pb is 15~26.49%), according to the liquid-solid ratio (mL:g) It is 5.7:1, and the acidity of sulfuric acid is 30g / L, at room temperature (18~22°C), and the reaction time is 2h. The leaching indicators are: the leaching rate of zinc from slag is 97.54%, the leaching rate of copper is 82.83%, and the leaching rate of arsenic is 73.05%.
[0054] 2). At 140°C, the mass ratio of iron to arsenic is 1.5:1, the arsenic concentration is 18g / l, the pH is 4.5, the copper ion concentration is 0.1g / L, the zinc concentration is 75 g / L, and the oxygen partial pressure is 1.3 Under the conditions of MPa and reaction time of 2 hours, scorodite was obtained by oxygen pressure precipitation of arsenic in acid leaching solution. Oxygen pressure arsenic precipitation indicators: copper precipitation rate 0.6%, arsenic precipitation rate 66%, ...
Embodiment 2
[0057] 1). Acid leaching the copper fumes to obtain an acid leaching solution. Take 700g of copper fume (Fe is 2~12%, As is 4~12%, Cu is 3~6%, Zn is 7~10%, Pb is 15~26.49%), according to the liquid-solid ratio (mL:g) It is 5.7:1, and the acidity of sulfuric acid is 22g / L, at room temperature (18~22°C), and the reaction time is 1.5h. The leaching indicators are: the leaching rate of zinc from slag is 91%, the leaching rate of copper is 78.21%, and the leaching rate of arsenic is 65.29%.
[0058] 2). At 150°C, the mass ratio of iron to arsenic is 1, the concentration of arsenic is 5g / l, the acidity of sulfuric acid is 10g / L, the concentration of copper ions is 2g / L, the concentration of zinc is 30 g / L, and the partial pressure of oxygen is 0.7MPa. Under the condition that the reaction time is 2.5 hours, the acid leaching solution is subjected to oxygen pressure precipitation to obtain scorodite. Indicators for oxygen pressure precipitation of arsenic: the precipitation rate of...
Embodiment 3
[0061] 1). Acid leaching the copper fumes to obtain an acid leaching solution. Take 700g of copper fume (Fe is 2~12%, As is 4~12%, Cu is 3~6%, Zn is 7~10%, Pb is 15~26.49%), according to the liquid-solid ratio (mL:g) It is 5.7:1, and the acidity of sulfuric acid is 14g / L, at room temperature (18~22°C), and the reaction time is 2h. The leaching indicators are: the leaching rate of zinc from slag is 86%, the leaching rate of copper is 76%, and the leaching rate of arsenic is 58.23%.
[0062] 2). At 160°C, the mass ratio of iron to arsenic is 2, the concentration of arsenic is 30g / l, the acidity of sulfuric acid is 20g / L, the concentration of copper ions is 8g / L, the concentration of zinc is 75 g / L, and the partial pressure of oxygen is 0.1 Under the conditions of MPa and reaction time of 3 hours, scorodite was obtained by oxygen pressure precipitation of arsenic in acid leaching solution. Indicators for oxygen pressure precipitation of arsenic: the precipitation rate of copper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com