Drug clinical test management system
A clinical trial and management system technology, applied in the system field of clinical trial subject management, can solve the problems of inability to transmit subject information in real time, drug inventory cannot be understood in real time, and clinical trials cannot be supervised in real time and efficiently , to achieve the effect of convenient and fast drug delivery, simplifying the work process and improving work efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0011] In order to deepen the understanding of the present invention, the present invention will be further described below in conjunction with the embodiments and accompanying drawings. The embodiments are only used to explain the present invention and do not constitute a limitation to the protection scope of the present invention.
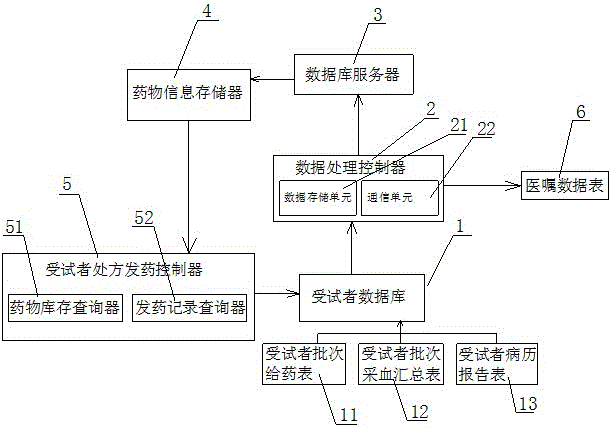
[0012] Such as figure 1 As shown, the drug clinical trial management system of the present invention includes a subject database 1 for collecting subject-related data, a data processing controller 2, a database server 3, a drug information storage 4 and a subject's prescription and drug delivery Controller 5, the subject's prescription drug delivery controller 5 includes a drug inventory queryer 51 and a drug delivery record queryer 52, and the subject database 1 includes a subject batch administration table 11, a subject batch blood collection summary Table 12 and subject medical record report form 13, the subject database 1 sends the collected ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com