Nasicon type sodium ion solid electrolyte material and preparation method thereof
A solid electrolyte, sodium ion battery technology, applied in the field of materials, can solve problems such as low ionic conductivity, and achieve the effects of simple preparation process, good safety performance, and excellent cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1 of the present invention provides a solid electrolyte material with a general chemical formula:
[0054] [Na 3+x-2y A y ][Zr 2-x m x ][Si 2-z M' z ]PO 12 ;
[0055] Among them, A is a divalent alkaline earth metal element that is doped and substituted for the Na site, specifically one or more of Ca, Sr, and Ba; M is an element that is doped and substituted for the octahedral Zr site, specifically La , one or more of Y, Al, In, Ga; M' is an element for doping and replacing the Si site, specifically one or two of Ge and Se;
[0056] Said x, y, and z are the molar percentages of the corresponding elements; wherein 0≤x≤0.3, 0≤y≤0.2, 0≤z≤0.3, and x, y, z are not 0 at the same time.
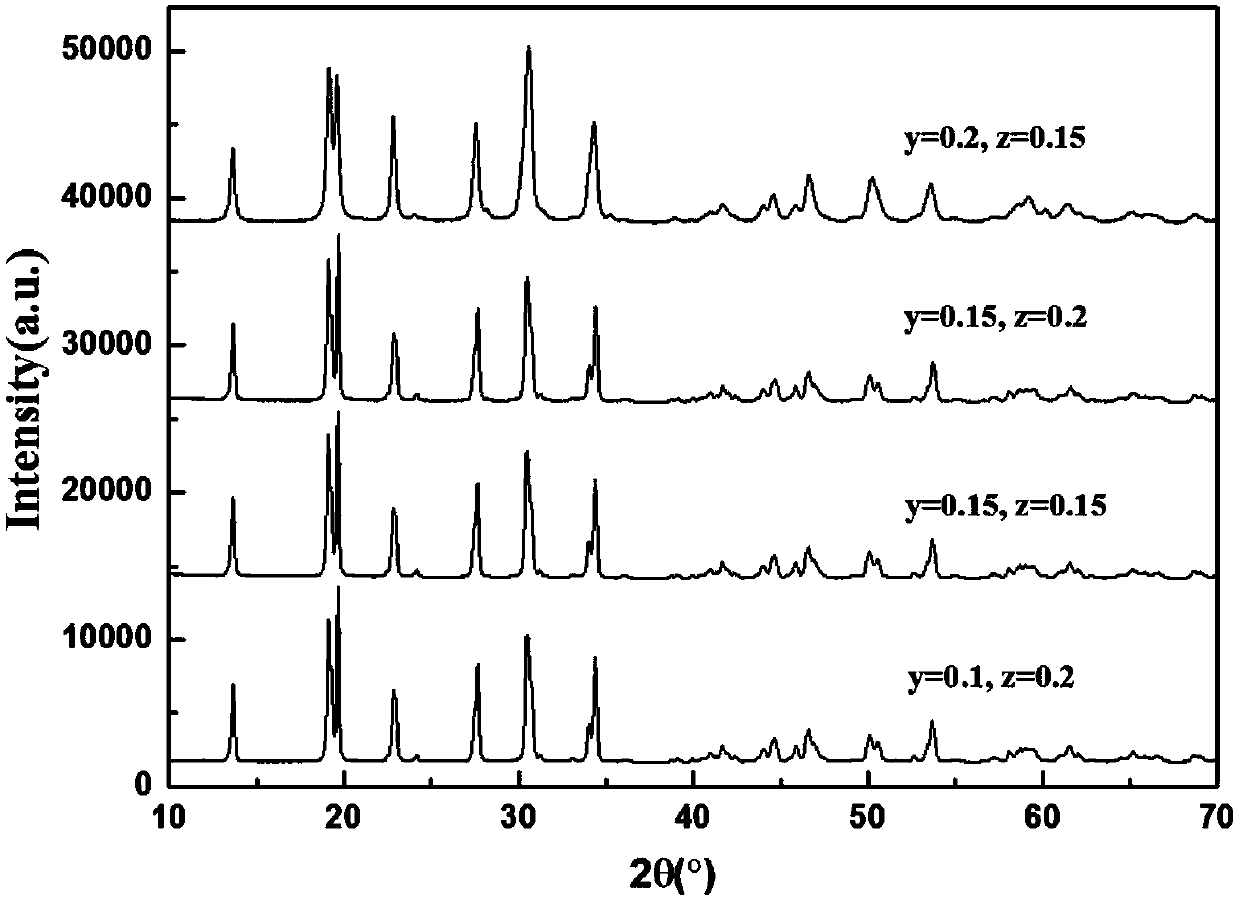
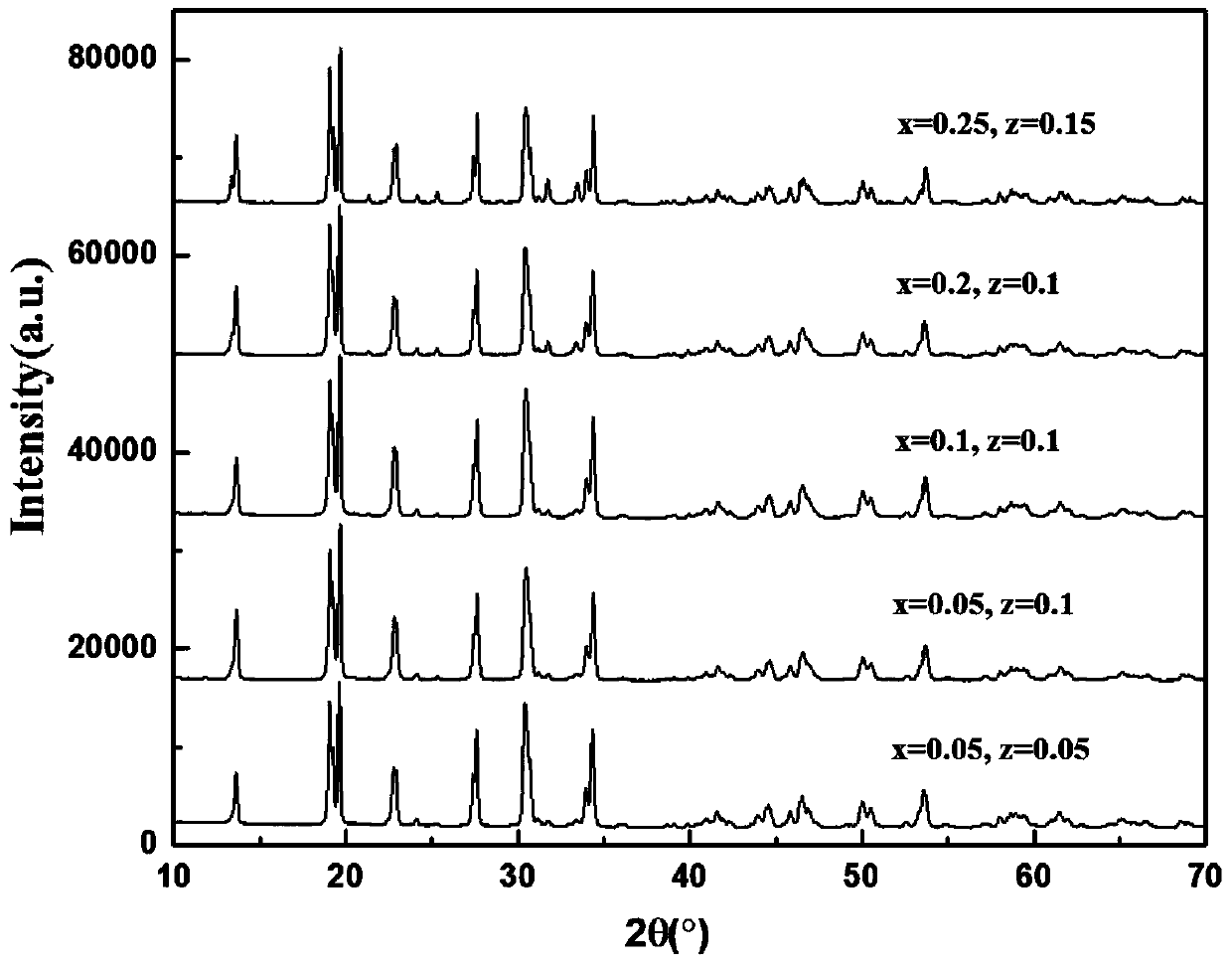
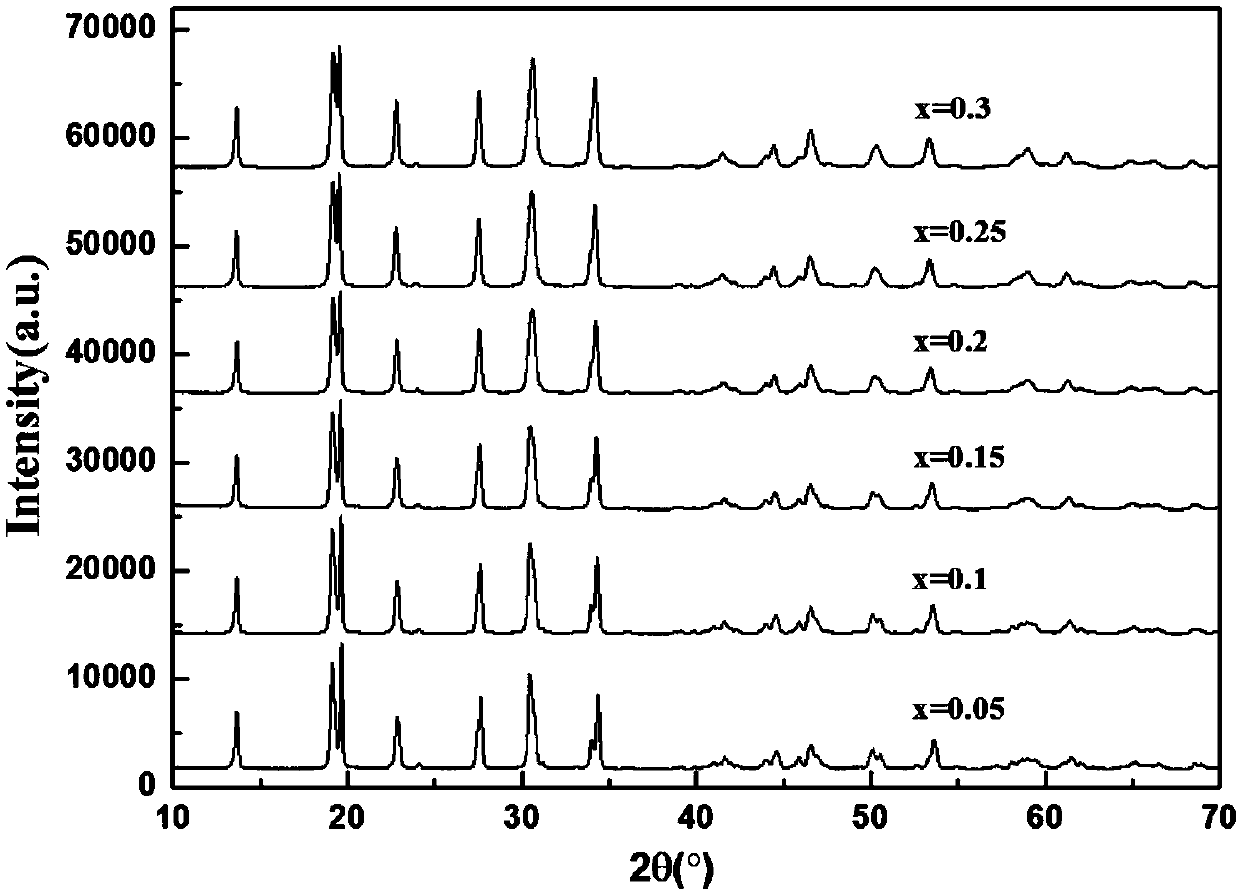
[0057] exist Figure 1-6 In , the X-ray diffraction (X-ray diffraction, XRD) patterns of NASICON sodium ion solid electrolyte materials doped with different concentrations of different elements are given.
[0058] The NASICON type sodium ion solid electrolyte material prov...
Embodiment 2
[0060] This embodiment provides a preparation method of a NASICON type sodium ion solid electrolyte material, specifically a solid phase method, such as Figure 7 shown, including:
[0061] Step 701, the sodium carbonate of required stoichiometric 110wt%~115wt% and the carbonate and / or nitrate of required stoichiometric A, ZrO 2 , SiO 2 、GeO 2 and / or SeO 2 , NH 4 h 2 PO 4 and / or (NH 4 ) 2 HPO 4 and M oxides are mixed in proportion to form a precursor;
[0062] Specifically, the A is specifically one or more of Ca, Sr, Ba, and the M is specifically one or more of La, Y, Al, Ga, In.
[0063] Step 702, using a ball milling method to uniformly mix the precursor to obtain a precursor powder;
[0064] Step 703, placing the precursor powder in a muffle furnace, and heat-treating it in an air atmosphere at 750° C. to 950° C. for 10 to 24 hours;
[0065] In step 704, the heat-treated precursor powder is ground and pressed into tablets.
[0066] Step 705, sintering in an ai...
Embodiment 3
[0069] This embodiment provides a preparation method of a NASICON type sodium ion solid electrolyte material, specifically a sol-gel method, such as Figure 8 shown, including:
[0070] Step 801, mix tetraethyl orthosilicate (TEOS), water, and ethanol at a molar ratio of 1:10:20, add the required stoichiometric germanium isopropoxide and citric acid, and stir at room temperature to 70°C For 1 hour, TEOS was hydrolyzed to form a mixed solution;
[0071] Step 802, the stoichiometric 110wt%~115wt% sodium nitrate or sodium acetate of required sodium, the nitrate and / or acetate of required stoichiometric A, zirconium oxynitrate, and the nitrate of M and / or Aqueous solution of acetate was added in the mixed solution successively, and then NH 4 h 2 PO 4 and / or (NH 4 ) 2 HPO 4 , to obtain a colorless and transparent sol;
[0072]Specifically, the A is specifically one or more of Ca, Sr, Ba, and M is specifically one or more of La, Y, Al, Ga, In;
[0073] Step 803, heating up ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com