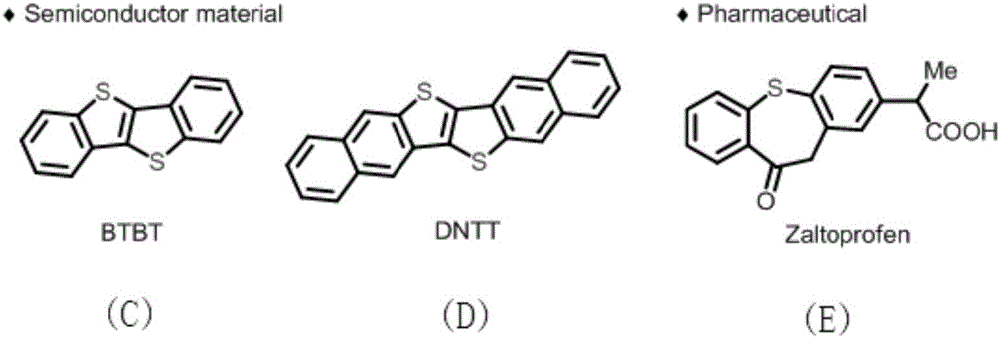
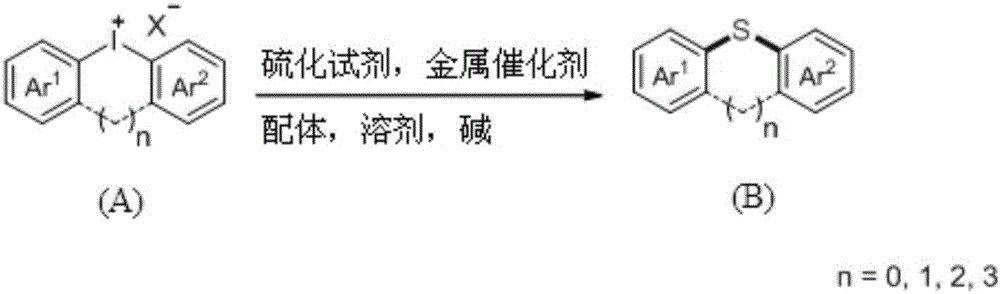
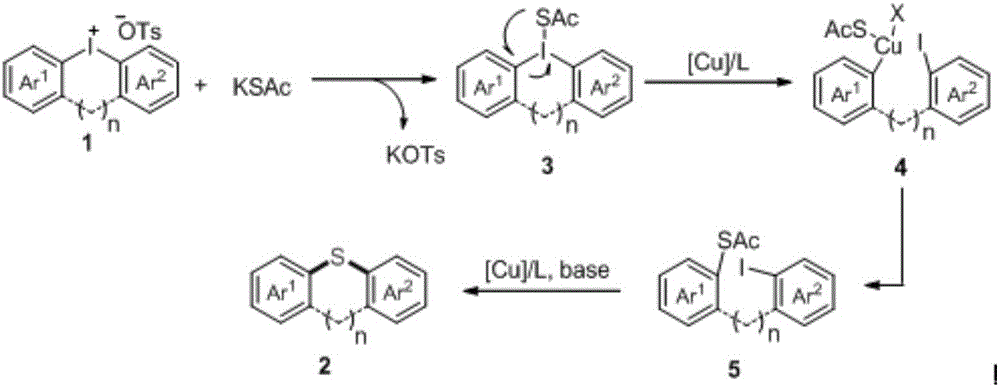
Diaryl thioether compound, and synthetic method and application thereof
A technology of diaryl sulfide and synthesis method, which is applied in the fields of sulfide preparation and organic chemistry, can solve the problems of incompatibility, unfavorable environmental protection of mercaptan, and poor atom economy of iodine salt, etc., and achieve good yield and low cost. The effect of wide applicability and simplified operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of compound 2a:
[0042]
[0043] Reaction formula (1)
[0044] Under nitrogen protection, the Cu(OTf) 2 (3.6mg, 0.01mmol), 1,10-phen (2.2mg, 0.012mmol), K 3 PO 4 (42.5mg, 0.2mmol), KSAc (22.8mg, 0.2mmol), periodinium salt 1a (56.1mg, 0.1mmol) and dry DMSO (1mL) were added to a dry Schlenk reaction tube. After the reaction was stirred at 100°C for 12 hours, it was lowered to room temperature, 10 mL of water was added to the system for dilution, and ethyl acetate (10 mL*3) was added for extraction, dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain a colorless Oil 2a (25.7 mg, 87%). 1 HNMR (400MHz, CDCl 3 ) 1 HNMR (400MHz, CDCl 3 )δ7.42(d, J=8.9Hz, 2H), 7.34(d, J=8.6Hz, 2H), 7.02(d, J=8.6Hz, 2H), 6.92(d, J=8.9Hz, 2H) ,3.83(s,3H); 13 CNMR (100MHz, CDCl 3 )δ160.2, 138.2, 135.6, 131.9, 129.5, 123.6, 119.4, 115.2, 55.4; -1 ;HRMS(EI)forC 13 h 11 OSBrCalculated: 293.9714, Found: 293.9718.
Embodiment 2
[0046] Synthesis of compound 2b:
[0047]
[0048] Reaction formula (2)
[0049] Under nitrogen protection, the Cu(OTf) 2 (3.6mg, 0.01mmol), 1,10-phen (2.2mg, 0.012mmol), K 3 PO 4 (42.5mg, 0.2mmol), KSAc (22.8mg, 0.2mmol), periodinium salt 1b (56.1mg, 0.1mmol) and dry DMSO (1mL) were added to a dry Schlenk reaction tube. After the reaction was stirred at 100°C for 12 hours, it was lowered to room temperature, 10 mL of water was added to the system for dilution, and ethyl acetate (10 mL*3) was added for extraction, dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain a colorless Oil 2b (20.7 mg, 70%). 1 HNMR (400MHz, CDCl 3 )δ7.34(d,J=8.4Hz,2H),7.19-7.12(m,2H),7.01-6.92(m,2H),6.83(d,J=8.4Hz,2H),3.73(s,3H ); 13 CNMR (100MHz, CDCl 3 )δ160.4, 141.6, 136.1, 130.2, 129.9, 128.5, 126.0, 123.0, 122.7, 115.3, 55.4; -1 ;HRMS(EI)forC 13 h 11 OSBrCalculated: 293.9714, Found: 293.9718.
Embodiment 3
[0051] Synthesis of compound 2c:
[0052]
[0053] Reaction formula (3)
[0054] Under nitrogen protection, the Cu(OTf) 2 (3.6mg, 0.01mmol), 1,10-phen (2.2mg, 0.012mmol), K 3 PO 4 (42.5mg, 0.2mmol), KSAc (22.8mg, 0.2mmol), periodonium salt 1c (51.7mg, 0.1mmol) and dry DMSO (1mL) were added to a dry Schlenk reaction tube. After the reaction was stirred at 100°C for 12 hours, it was lowered to room temperature, 10 mL of water was added to the system for dilution, and ethyl acetate (10 mL*3) was added for extraction, dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain a colorless Oil 2c (16.8 mg, 67%). 1 HNMR (400MHz, CDCl 3 )δ7.43(d, J=7.3Hz, 2H), 7.22(d, J=7.1Hz, 2H), 7.10(d, J=7.1Hz, 2H), 6.93(d, J=7.3Hz, 2H) ,3.82(s,3H); 13 CNMR (100MHz, CDCl 3 )δ160.1, 137.4, 135.5, 131.6, 129.3, 129.0, 123.8, 115.2, 55.4; -1 ;HRMS(EI)forC 13 h 11 OSCl Calculated: 250.0219, Found: 250.0222.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com