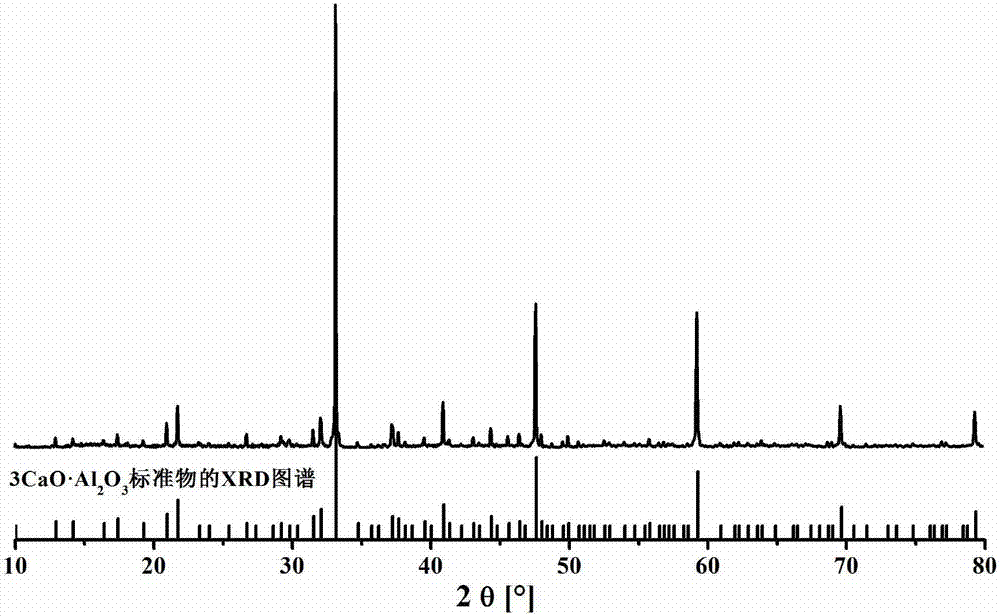
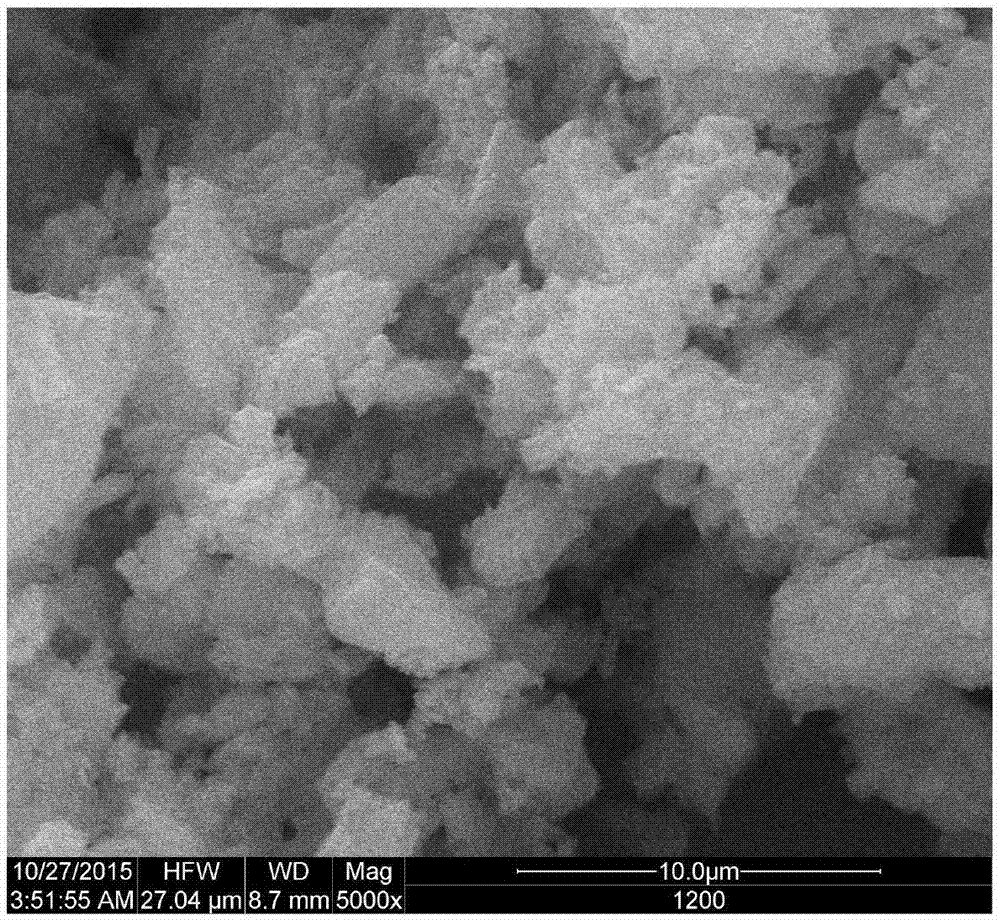
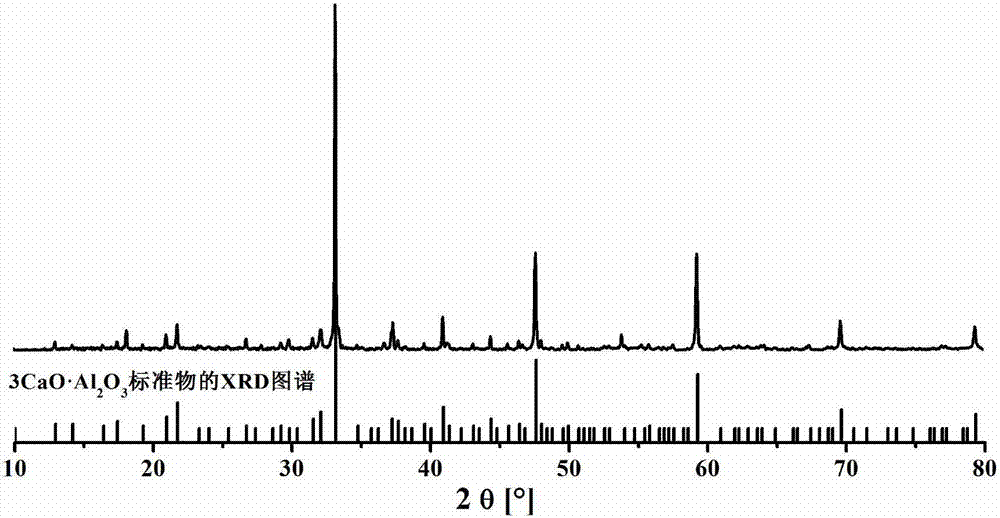
A method for preparing tricalcium aluminate rapidly and with low energy consumption
A technology of tricalcium aluminate and low energy consumption, applied in the preparation of calcium aluminate, alkaline earth metal aluminate/alumina/aluminum hydroxide, etc., can solve the problem of uneven mixing of reactants, difficulty in ion diffusion, complicated synthesis steps, etc. problems, to achieve the effect of shortening the preparation cycle, reducing technical requirements, and simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Objective: To prepare 20.00g of tricalcium aluminate.
[0047] The oxide form 3CaO Al according to the chemical formula of tricalcium aluminate 2 o 3 , the calculated CaO content is 62.27%, Al 2 o 3 The content is 37.73%. According to the mass of tricalcium aluminate to be prepared is 20.00g, the mass of CaO in the sample after sintering is calculated to be 20.00×62.27%=12.45g.
[0048] According to the conservation of Ca element mass, the analytically pure calcium nitrate tetrahydrate Ca(NO 3 ) 2 4H 2 O requirement is 12.45×4.21=52.41g;
[0049] Analytical pure calcium nitrate tetrahydrate Ca(NO 3 ) 2 4H 2 1.06 times of the O mass calculates the required analytical pure aluminum nitrate nonahydrate Al(NO 3 ) 3 9H 2 The mass of O is 52.41×1.06=55.56g.
[0050] Calculation and analysis of pure calcium nitrate tetrahydrate Ca(NO 3 ) 2 4H 2 O and analytically pure aluminum nitrate nonahydrate Al(NO 3 ) 3 9H 2 The gross mass of O is 52.41+55.56=107.97g, ...
Embodiment 2
[0057] Purpose: To prepare 15.00 g of tricalcium aluminate.
[0058] The oxide form 3CaO Al according to the chemical formula of tricalcium aluminate 2 o 3 , the calculated CaO content is 62.27%, Al 2 o 3 The content is 37.73%. According to the mass of tricalcium aluminate to be prepared is 15.00g, the mass of CaO in the sample after sintering is calculated to be 15.00×62.27%=9.34g.
[0059] According to the conservation of Ca element mass, the analytically pure calcium nitrate tetrahydrate Ca(NO 3 ) 2 4H 2 O requirement is 9.34×4.21=39.32g;
[0060] Analytical pure calcium nitrate tetrahydrate Ca(NO 3 ) 2 4H 2 1.06 times of the O mass calculates the required analytical pure aluminum nitrate nonahydrate Al(NO 3 ) 3 9H 2 The mass of O is 39.32×1.06=41.68g.
[0061] Calculation and analysis of pure calcium nitrate tetrahydrate Ca(NO 3 ) 2 4H 2 O and analytically pure aluminum nitrate nonahydrate Al(NO 3 ) 3 9H 2 The gross mass of O is 39.32+41.68=81.00g, calc...
Embodiment 3
[0068] Purpose: To prepare 10.00 g of tricalcium aluminate.
[0069] The oxide form 3CaO Al according to the chemical formula of tricalcium aluminate 2 o 3 , the calculated CaO content is 62.27%, Al 2 o 3 The content is 37.73%. According to the mass of tricalcium aluminate to be prepared is 10.00g, the mass of CaO in the sample after sintering is calculated to be 10.00×62.27%=6.23g.
[0070] According to the conservation of Ca element mass, the analytically pure calcium nitrate tetrahydrate Ca(NO 3 ) 2 4H 2 O requirement is 6.23×4.21=26.23g;
[0071] Analytical pure calcium nitrate tetrahydrate Ca(NO 3 ) 2 4H 2 1.06 times of the O mass calculates the required analytical pure aluminum nitrate nonahydrate Al(NO 3 ) 3 9H 2 The mass of O is 26.23×1.06=27.80g.
[0072] Calculation and analysis of pure calcium nitrate tetrahydrate Ca(NO 3 ) 2 4H 2 O and analytically pure aluminum nitrate nonahydrate Al(NO 3 ) 3 9H 2 The gross mass of O is 26.23+27.80=54.03g, calcul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com