Method for the determination of soluble gpc3 protein
A variable and variable region technology, applied in immunoglobulin, chemical instruments and methods, specific peptides, etc., can solve the problem of 5-year survival rate retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0177]In the mutant GPC3 core (core) formed by converting the serines of the two heparan sulfate binding sites (positions 495 and 509) of human glypican 3 (GPC3: SEQ ID NO: 70) into alanine The C-terminus of the extracellular domain (position 25-563) is endowed with a His tag to obtain the GPC3 core (sGPC3-His) protein, and the GPC3 core (sGPC3-His) protein is used for Balb / c or MRL / lpr mice A total of 6 to 9 immunizations were performed, and 3 days after the final immunization, mouse spleen cells were fused with mouse myeloma cells SP2 / 0 by a conventional method using PEG1500 or HVJ-E (Ishihara Sangyo).
[0178] Next, hybridoma cells were tested using ELISA in which sGPC3-His was directly immobilized, ELISA in which anti-His antibody was immobilized and sGPC3-His was bound, or cell ELISA (Cell ELISA) using CHO cells that constantly express GPC3. screening (see WO 2006 / 006693).
Embodiment 2
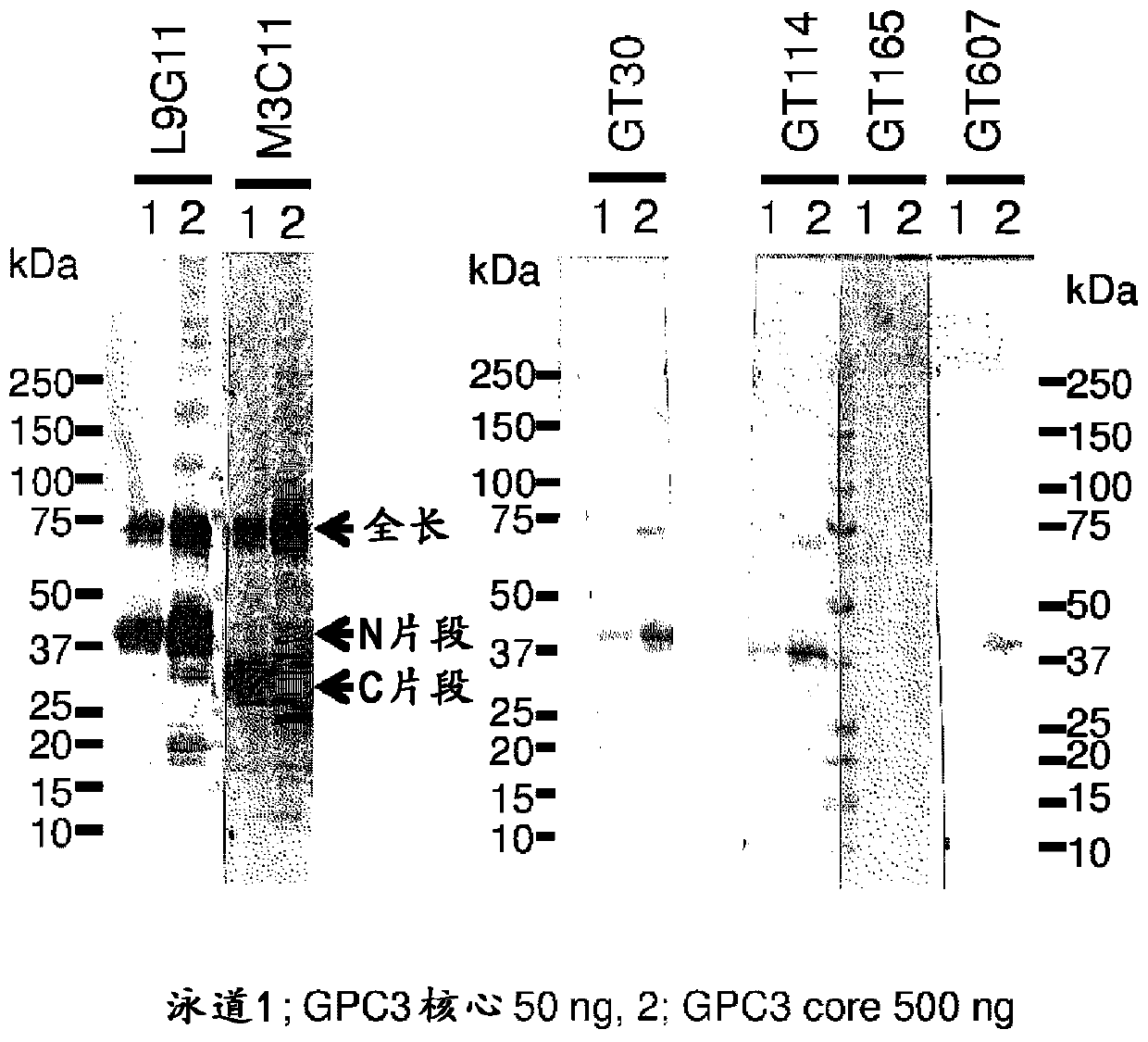
[0180] It has been found that the sGPC3-His protein can be seen in SDS-PAGE under reducing conditions with bands of about 86kDa, about 43kDa, and about 33kDa, which are the full-length type, the N-terminal fragment obtained by protease cleavage, and the C-terminal Fragment (WO2006 / 006693). Using the mouse antibody obtained through the above screening, perform Western blotting against sGPC3-His, and select an antibody that does not recognize the C-terminal fragment. As a result, GT30, GT114, GT607 as N-terminal fragment recognition antibodies, and GT165 ( figure 1 ).
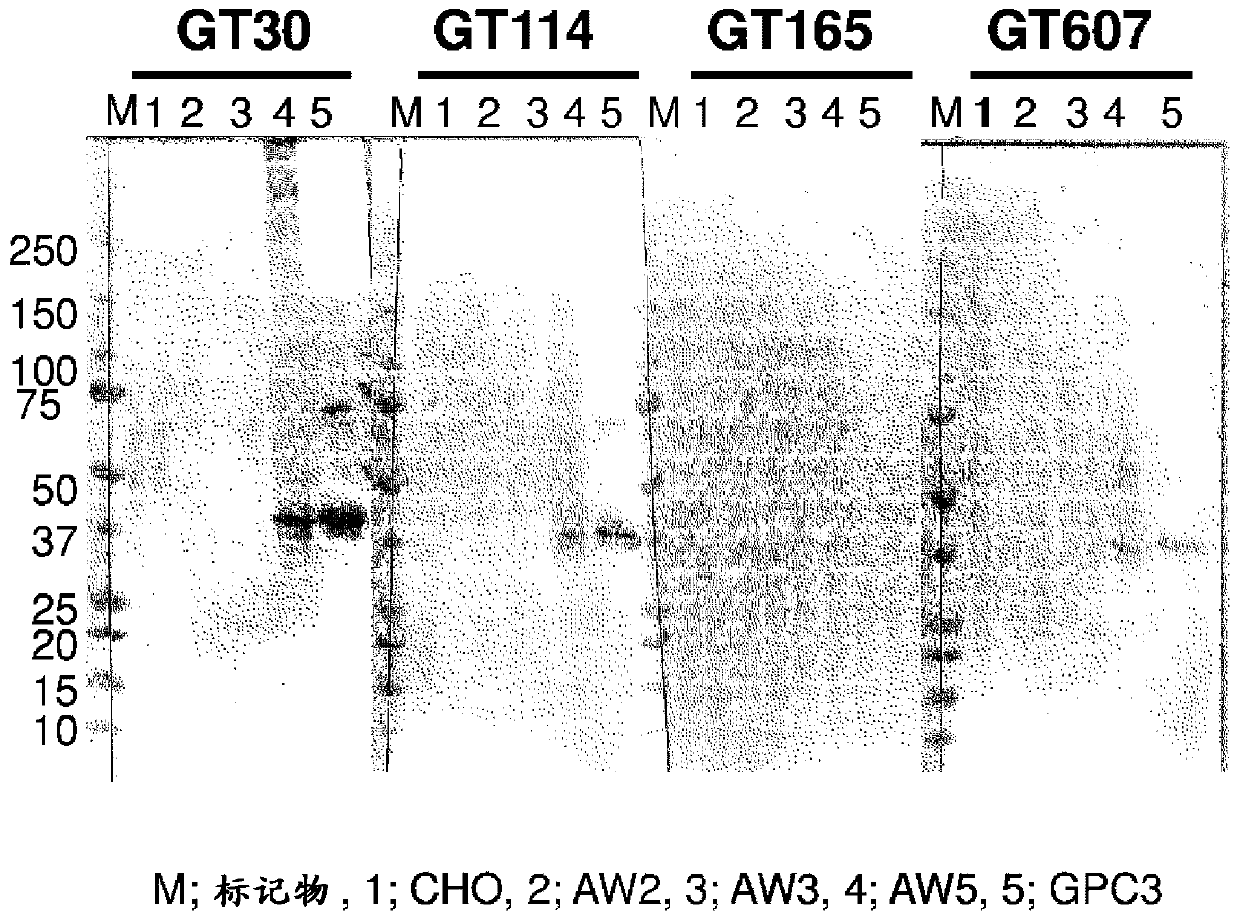
[0181] Next, in CHO cells, recombinant products with His tags attached to each of the C-terminals of GPC3 from 1 to 196 (AW2), 1 to 218 (AW3), and 1 to 357 (AW5) were expressed, and each CHO cells were pelleted for Western blotting. results, such as figure 2 , as shown in Table 1, although reactivity with AW5 or sGPC3-His was confirmed for GT30, GT114, and GT607, reactivity was not confirmed for AW2 and AW3,...
Embodiment 3
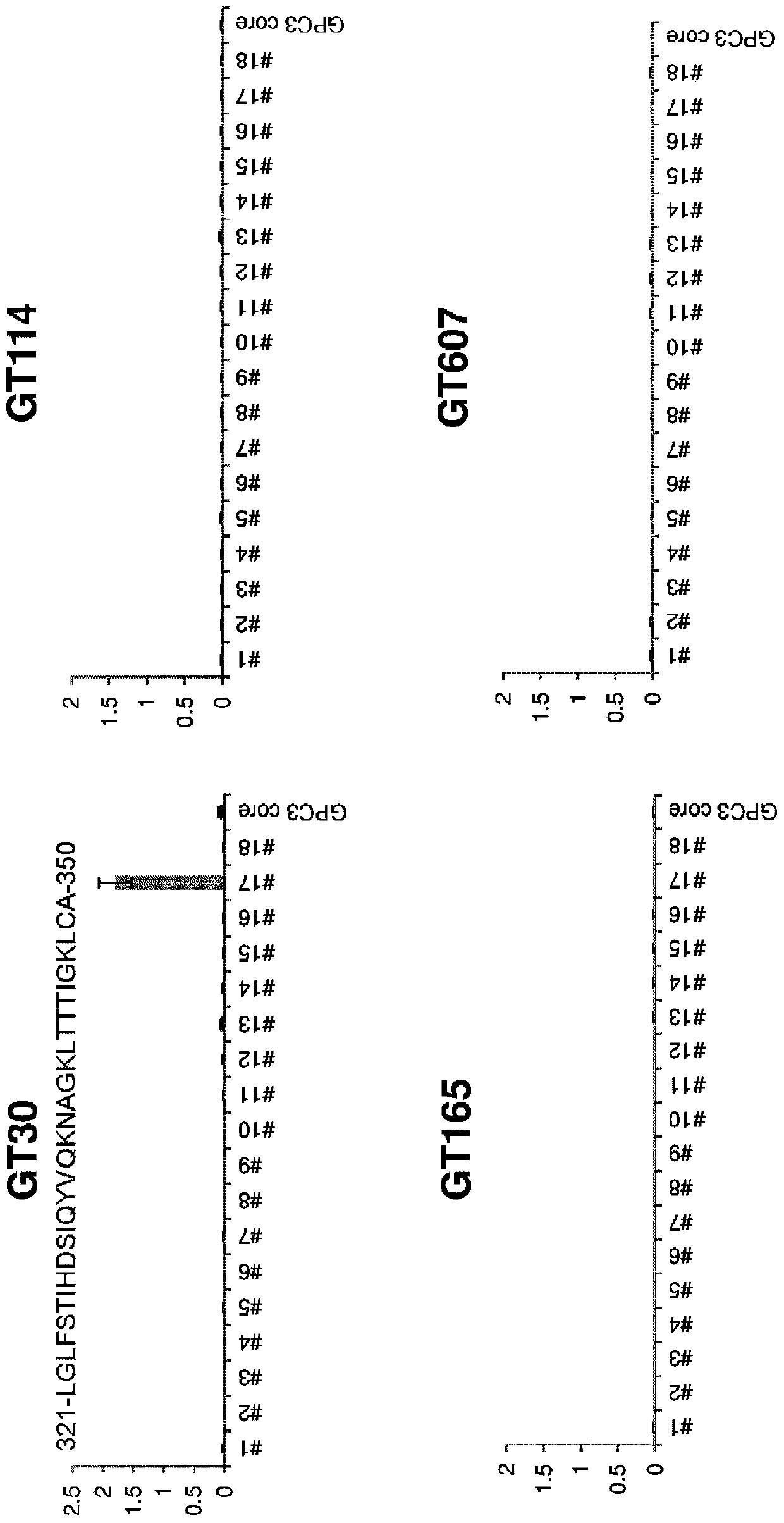
[0185] In order to identify the epitope of the above-mentioned antibody in more detail, the amino acid sequence of GPC3 was divided into 30-residue overlapping peptides containing 10 residues, and the corresponding peptides were synthesized (SEQ ID NO: 1-29 in Table 2). After dissolving the synthetic peptide in dimethyl sulfoxide so as to be 1 mg / mL, it was diluted to 1 μg / mL with PBS. The diluted peptide solution was added to a 96-well plate in an amount of 70 μL per well, and immobilized at room temperature for 1 hour. Unimmobilized peptides were removed, and blocking buffer 20% Blocking-one (Nacalai Tesque, Inc.) was added in an amount of 200 μL per well for overnight blocking. After the blocking buffer was removed and washed three times with TBS-T, an antibody (diluted to 2 µg / mL with blocking buffer) was added in an amount of 70 µL / well. One hour later, the antibody was removed, TBS-T was added in an amount of 200 μL per well, and washed three times. The HRP-conjugated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com