A dry heat treatment stabilizer for preparing human prothrombin complex and its application
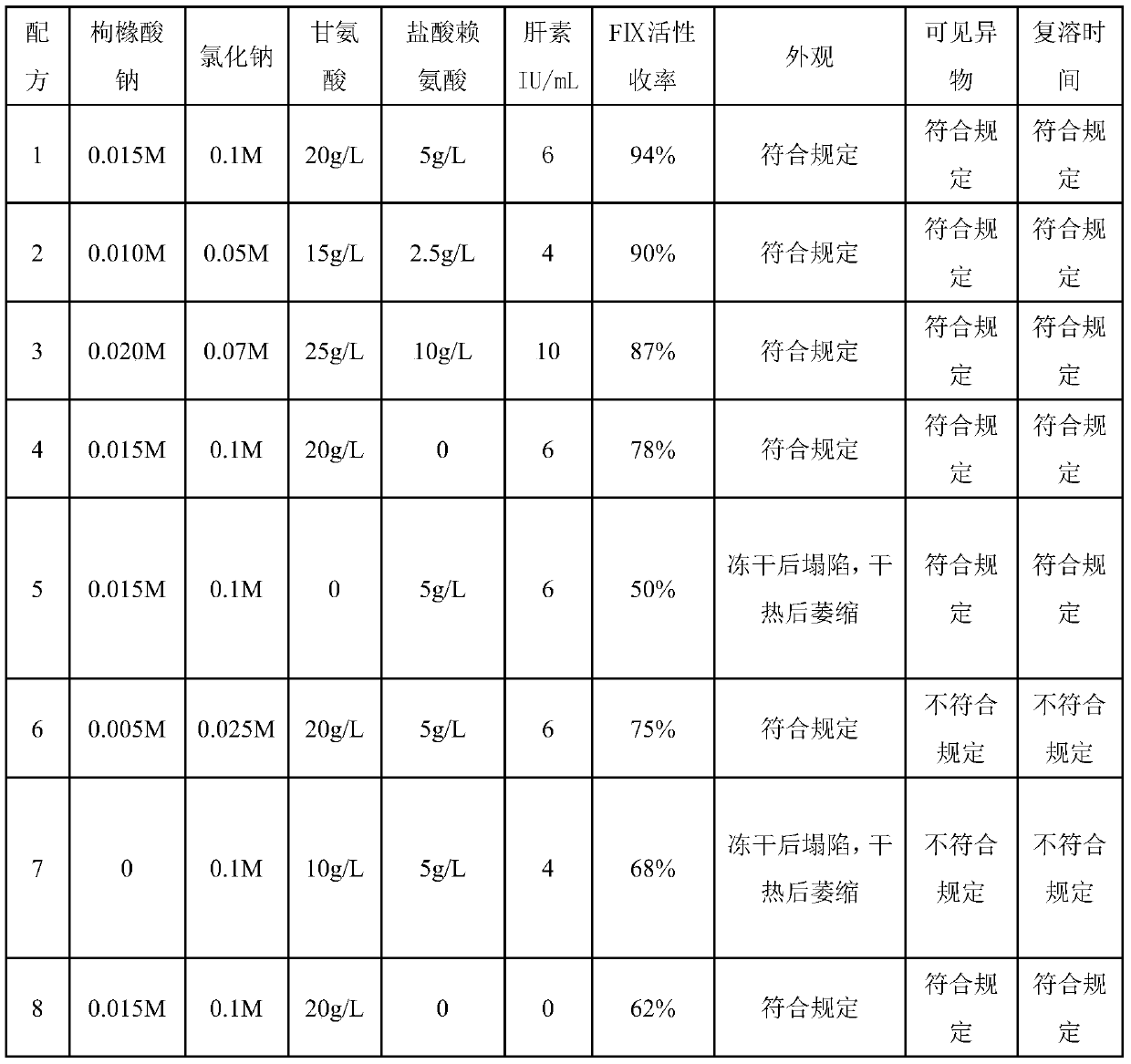
A technology of human prothrombin and dry heat treatment, which is applied in the field of dry heat treatment stabilizer for the preparation of human prothrombin complex, which can solve the problem of poor protection effect of coagulation factors, only 78% FIX activity yield, and low practical application value, etc. problems, to achieve good industrial application prospects, quality, safety and effectiveness, and to maintain the effect of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 Preparation of human prothrombin complex of the present invention
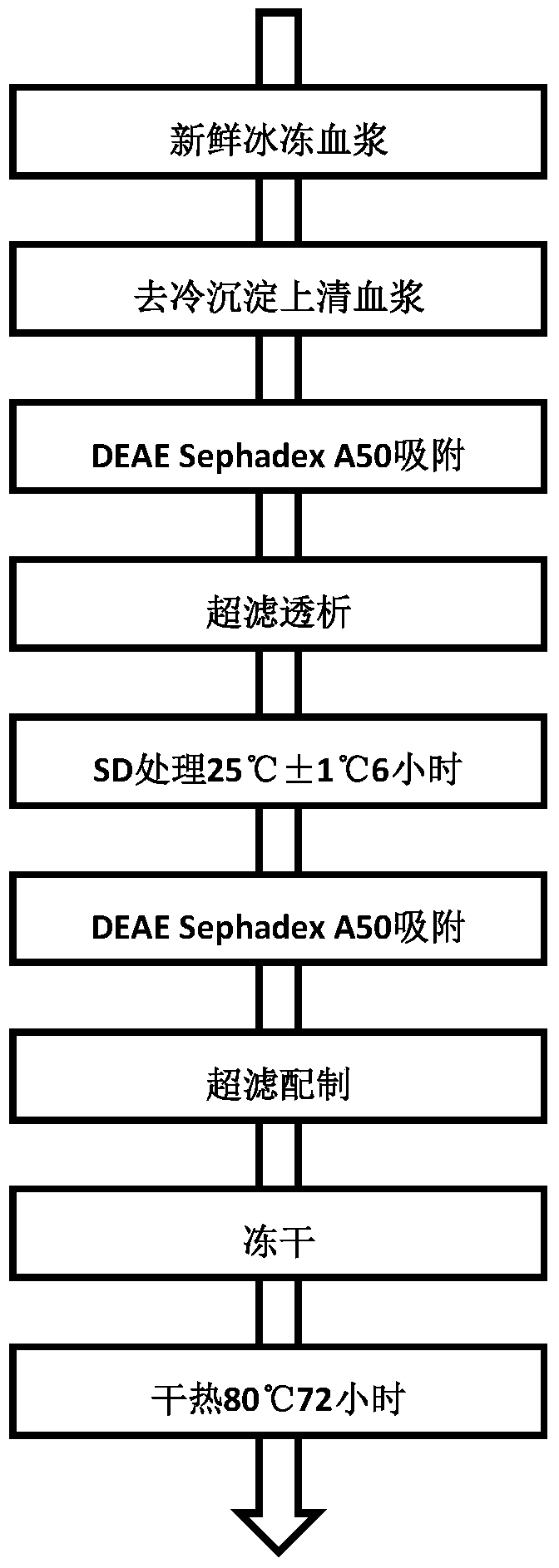
[0027] The preparation process of human prothrombin complex of the present invention is shown in figure 1 .
[0028] The preparation method is as follows:
[0029] Ⅰ. Separation and purification
[0030] (1) Using DEAE Sephadex A50 ion exchange chromatography for the first time, using DEAE Sephadex A50 as the starting material, collecting the eluate to obtain the first purified product;
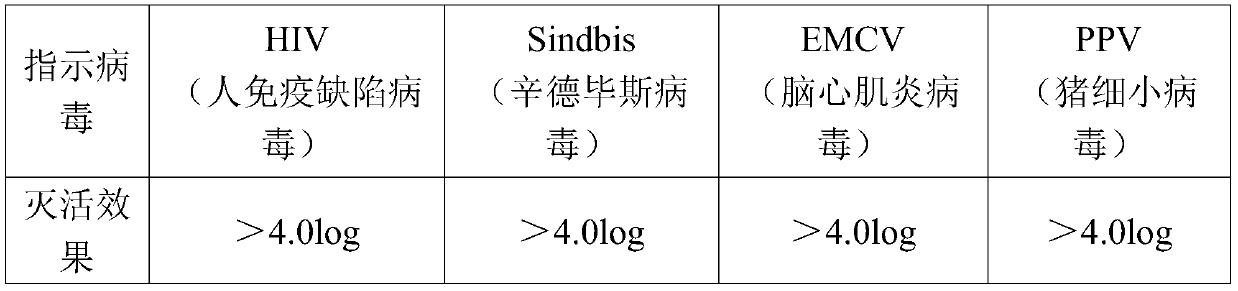
[0031] (2) After ultrafiltration and dialysis for the first purified product, Tween-80 and tributyl phosphate were added to make the final concentrations 1% and 0.3% respectively, and treated at 25°C for 6 hours to complete the first virus inactivation (i.e. S / D virus inactivation);
[0032] (3) Take the product after the first virus inactivation and use DEAE Sephadex A50 for the second ion exchange chromatography, collect the eluate, and obtain the second purified product (that is, the eluate containi...
Embodiment 2
[0036] Embodiment 2 Preparation of human prothrombin complex of the present invention
[0037] The preparation method is as follows:
[0038] Ⅰ. Separation and purification
[0039] (1) Using DEAE Sephadex A50 ion exchange chromatography for the first time, using DEAE Sephadex A50 as the starting material, collecting the eluate to obtain the first purified product;
[0040] (2) After ultrafiltration and dialysis for the first purified product, Tween-80 and tributyl phosphate were added to make the final concentrations 1% and 0.3% respectively, and treated at 25°C for 6 hours to complete the first virus inactivation (i.e. S / D virus inactivation);
[0041] (3) Take the product after the first virus inactivation and use DEAE Sephadex A50 for the second ion exchange chromatography, collect the eluate, and obtain the second purified product (that is, the eluate containing the human prothrombin complex);
[0042] Ⅱ. Preparation
[0043] (1) Carry out ultrafiltration dialysis to ...
Embodiment 3
[0045]Embodiment 3 Preparation of human prothrombin complex of the present invention
[0046] The preparation method is as follows:
[0047] Ⅰ. Separation and purification
[0048] (1) Using DEAE Sephadex A50 ion exchange chromatography for the first time, using DEAE Sephadex A50 as the starting material, collecting the eluate to obtain the first purified product;
[0049] (2) After ultrafiltration and dialysis for the first purified product, Tween-80 and tributyl phosphate were added to make the final concentrations 1% and 0.3% respectively, and treated at 25°C for 6 hours to complete the first virus inactivation (i.e. S / D virus inactivation);
[0050] (3) Take the product after the first virus inactivation and use DEAE Sephadex A50 for the second ion exchange chromatography, collect the eluate, and obtain the second purified product (that is, the eluate containing the human prothrombin complex);
[0051] Ⅱ. Preparation
[0052] (1) Carry out ultrafiltration dialysis to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com