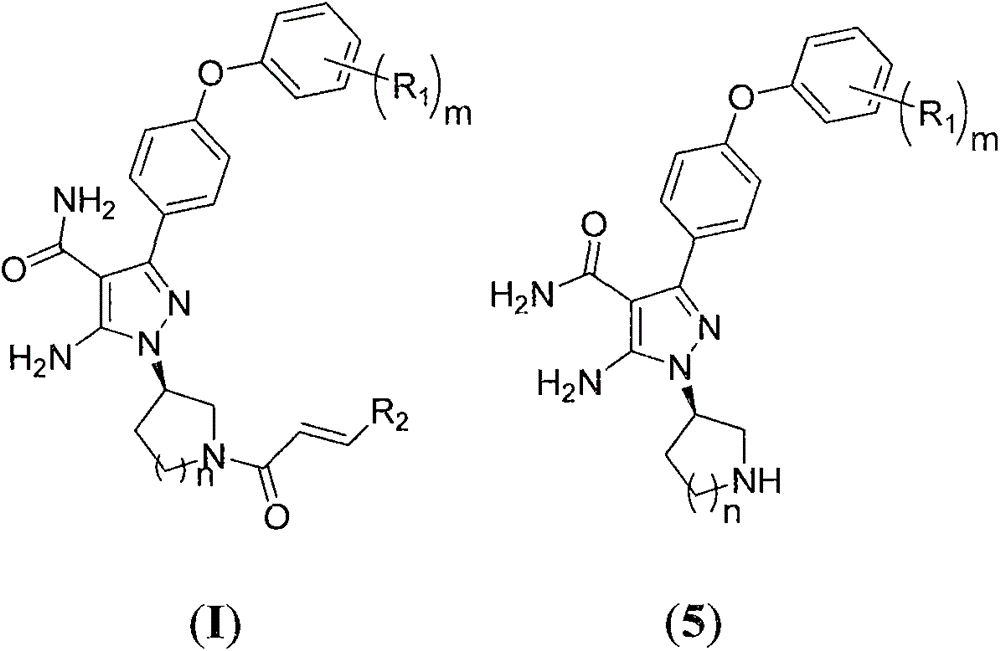
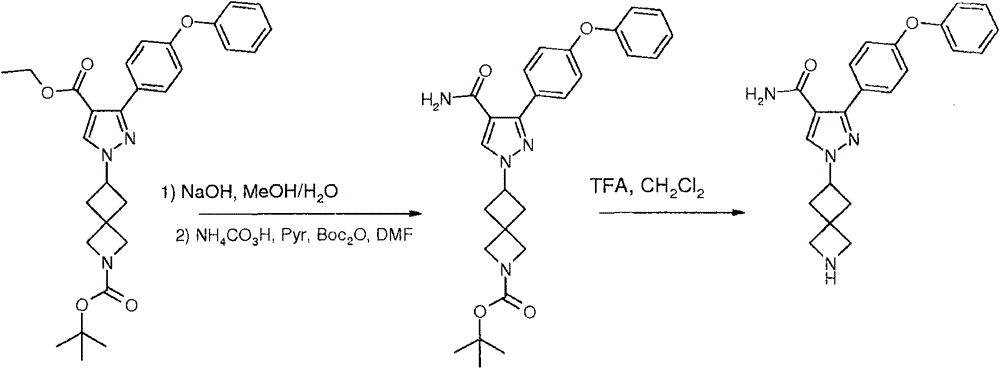
Preparation method for preparing Bruton's tyrosine kinase (BTK) inhibitor
A technology of acidic conditions and compounds, applied in organic chemistry and other fields, can solve problems such as incomplete reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
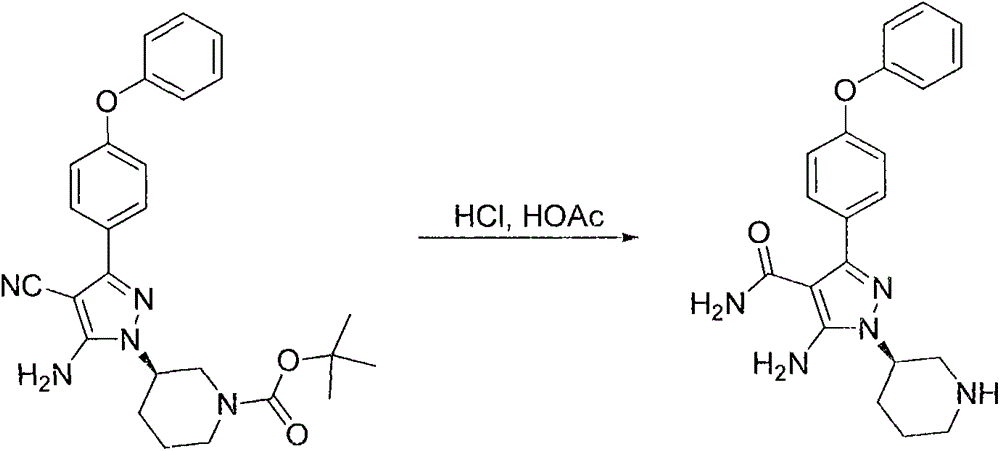
[0085] Example 1: (R)-3-(5-amino-4-cyano-3-(4-phenoxyphenyl)-1H-pyrazol-1-yl)tetrahydropyrrole-1-carboxylic acid tert-butyl Preparation of ester (compound 3a)
[0086]
[0087] Add compound 1a (600g, 2.17mol), compound 2a (815g, 2.39mol), powdery cesium carbonate (1417g, 4.35mol) and N, N-methylformamide (DMF, 6L), mechanically stirred, heated to 80°C (about 1.5 hours), and sent to HPLC to monitor the reaction after 2 hours. After the reaction was completed, stop heating, cool to room temperature, then add 10L ethyl acetate and 15L water, and stir for 30 Minutes (rotating speed 200), leave standstill, separate liquid, water phase is discarded, and organic phase is stirred dry with 1kg anhydrous sodium sulfate machine, filters, and filtrate is concentrated to obtain deep reddish-brown oily matter, and this crude product is recrystallized with dioxane dioxane Once, 450 g of a light yellow powdery solid was obtained, with a yield of 46.4%.
[0088] 1 H NMR (400MHz, CDCl 3 ...
Embodiment 2
[0089] Example 2: (R)-5-amino-3-(4-phenoxyphenyl)-1-(tetrahydropyrrol-3-yl)-1H-pyrazole-4-carbonitrile hydrochloride (compound 4a ) preparation
[0090]
[0091] Compound 3a (463 g) and 6 L of dichloromethane were added to a dry 10 L three-necked flask. After dissolving, the temperature was cooled to 5° C. in an ice-water bath, and dry HCl gas was introduced for 1 hour, then stirred overnight. Suction filter, wash once with 1 L of dichloromethane, and dry in the air to obtain 385 g of light yellow powdery solid (97.0%).
[0092] 1H NMR (400MHz, DMSO): δ9.36-9.67 (2H, brs), 7.86 (2H, d, J = 8.8HZ), 7.40 (2H, t, J = 8.0Hz), 7.16 (1H, t, J =7.6Hz), 7.08(2H, d, J=8.8Hz), 7.04(2H, d, J=8.0Hz), 6.94(2H, s), 5.10-5.13(1H, m), 3.39-3.58(4H , m), 2.25-2.34 (1H, m), 2.13-2.20 (1H, m).
Embodiment 3
[0093] Example 3: (R)-5-amino-3-(4-phenoxyphenyl)-1-(tetrahydropyrrol-3-yl)-1H-pyrazole-4-carboxamide (compound 5a) preparation
[0094]
[0095] Add 3.8kg of polyphosphoric acid and 380mL of deionized water to a dry 10L three-necked flask in turn, stir and dilute, heat up to an internal temperature of 80°C, and add compound 4a (380g, 0.995mol) in batches. After the addition is complete, The internal temperature rose to 90-95°C, stirred for 8 hours, and sent to HPLC to monitor the reaction. After the reaction was completed, after the temperature dropped to room temperature, the viscous reaction liquid in the reaction bottle was poured into ice water in batches, and after pouring, While adding crushed ice, sodium hydroxide solution was added, and the addition was stopped when the pH was adjusted to 5-6. The resulting white powdery solid was filtered with suction, washed with water twice (1 L / time), and dried to obtain a light yellow foamy solid 5a (350 g , 96.8%).
[0096]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com