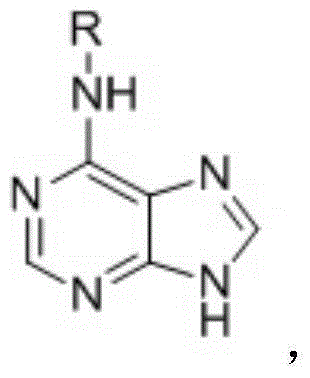
6-substitued aminopurine compound acting on EGFR sensitive mutation kinase EGFR<L858R> and EGFR<(d746-750)> and application of 6-substitued aminopurine compound
An aminopurine and compound technology, applied in the field of medicinal chemistry, can solve the problems of large side reactions and low effect of EGFR inhibitor, and achieve the effect of high-efficiency treatment of tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
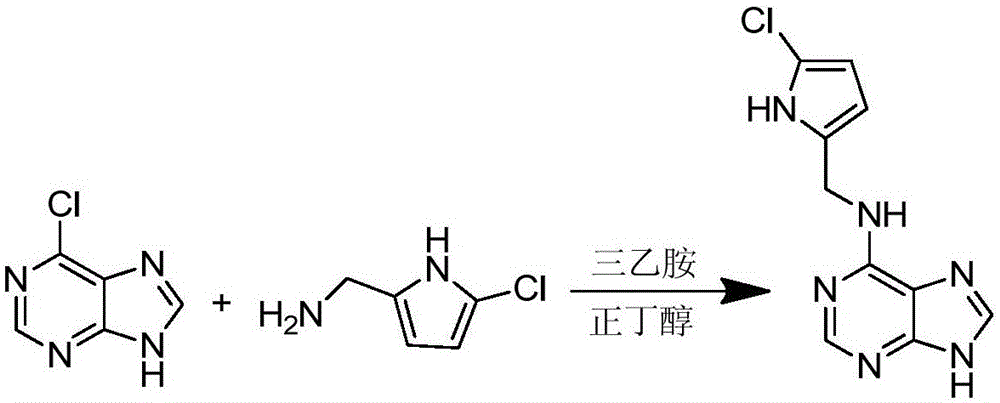
[0024] The synthesis of embodiment 1 compound 6-(5-chloro-pyrrole-2-methylamino)purine (A1)
[0025] 1 Synthetic route of the compound
[0026] The specific synthetic route is as figure 1 shown.
[0027] 2 synthetic steps
[0028] 2.1 Preparation of 6-(5-chloro-pyrrole-2-methylamino)purine (A1)
[0029] Measure 5ml of n-butanol, weigh 80mg of 6-chloropurine and 100mg of (5-chloro-1H-pyrrol-2-yl)methylamine, put them into a 25ml round bottom flask, and add 50μl of triethyl Amines act as catalysts and are added to a stir bar. Heated to reflux in an oil bath at 110°C for 5h, cooled, until a white solid precipitated out, filtered under reduced pressure, washed twice with n-butanol, and dried to obtain 6-(5-chloro-pyrrole-2-methylamino)purine (A1 ).
[0030] 2.2 Physical characteristics of 6-(5-chloro-pyrrole-2-methylamino)purine (A1)
[0031] 1 H-NMR (DMSO-d 6 )δ(ppm): 12.987(s,1H,7'or 9'-Purine-H), 8.220(s,1H,-NH), 8.140-8.15(m,2H,2',8'-Purine-H ), 7.858(s,1H,1'-Pyrrole...
Embodiment 2
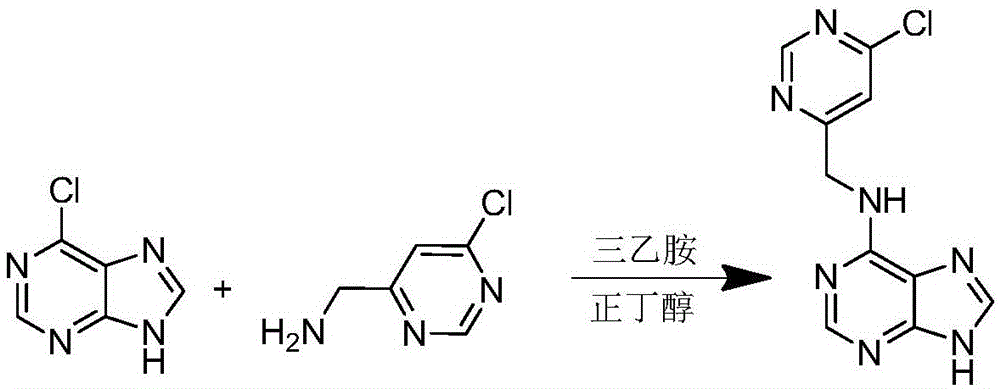
[0032] The synthesis of embodiment 2 compound 6-(6-chloro-pyrimidine-4-methylamino)purine (A2)
[0033] 1 Synthetic route of the compound
[0034] The specific synthetic route is as figure 2 shown.
[0035] 2 synthetic steps
[0036] 2.1 Preparation of 6-(6-chloro-pyrimidine-4-methylamino)purine (A2)
[0037] Measure 5ml of n-butanol, weigh 80mg of 6-chloropurine and 120mg of (6-chloropyrimidin-4-yl)methylamine, put them into a 25ml round bottom flask, and add 50μl of triethylamine as a catalyst , add a stir bar. Heated to reflux in an oil bath at 110°C for 5h, cooled until a white solid precipitated out, filtered under reduced pressure, washed twice with n-butanol, and dried to obtain 6-(6-chloro-pyrimidine-4-methylamino)purine (A2 ).
[0038] 2.2 Physical characteristics of 6-(6-chloro-pyrimidine-4-methylamino)purine (A2)
[0039] 1 H-NMR (DMSO-d 6 )δ(ppm): 13.276(s,1H,7'or 9'-Purine-H), 9.432(s,1H,2'-Pyrimidine-H), 8.720(s,1H,-NH-), 8.125- 8.243(m,2H,2',8'-Purine...
Embodiment 3
[0040] Example 3 compound in vitro EGFR inhibitory activity test
[0041] Screening of EGFR kinase activity in vitro: The method used in the experiment is Caliper Mobility Shift Assay, which is a detection platform based on the mobility detection technology of microfluidic chip technology. The specific experimental steps are: configure 1.25x kinase reaction buffer (62.5mmol / L HEPES, pH7.5; 0.001875% Brij-35; 12.5mmol / L MgCl 2; 2.5mmol / L DTT) and kinase reaction stop solution (100mmol / L HEPES, pH7.5; 0.015% Brij-35; 0.2% Coating Reagent#3); in 5μl of 5x concentration compound solution (DMSO dissolved, diluted with water 10 times) add 10 μl of 2.5x EGFR kinase solution (add kinase in 1.25x kinase reaction buffer), react at room temperature for 10 minutes, then add 10 μl of 2.5x substrate peptide solution (add FAM in 1.25x kinase reaction buffer labeled peptide and ATP), after reacting at 28°C for a specific time, 25 μl of kinase reaction stop solution was added. The data was c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com