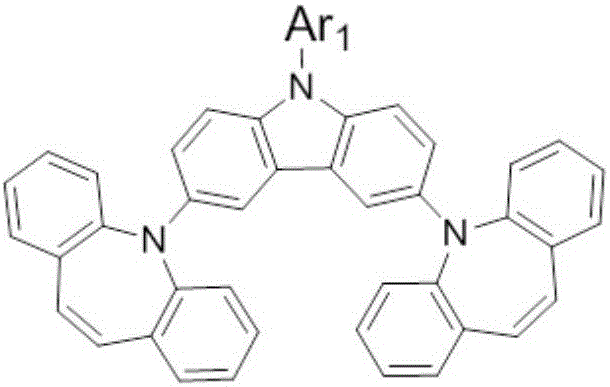
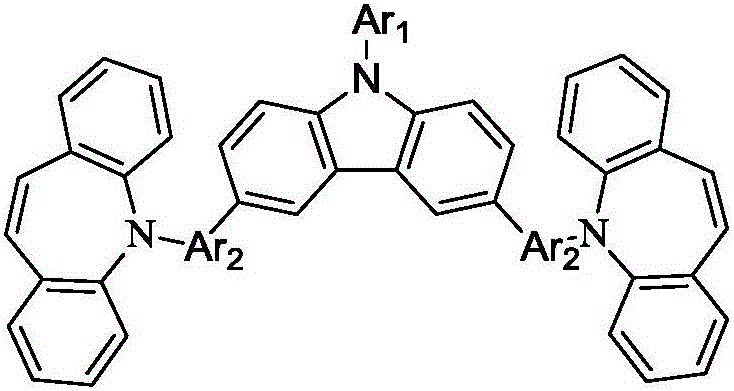
Organic photoelectric material with carbazole and iminostilbene structure and application of organic photoelectric material
An organic optoelectronic material, iminostilbene technology, applied in the direction of organic chemistry, circuits, electrical components, etc., to achieve the effect of reducing the lighting voltage, high-efficiency electroluminescence performance, and high fluorescence quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1, preparation organic optoelectronic material
Embodiment 1-1
[0051] The preparation of embodiment 1-1 intermediate A-1
[0052] Under nitrogen protection, dissolve N-phenylcarbazole (48.6g, 0.2mol) in 500mL DMF (N,N-dimethylformamide) in a 1L three-necked flask, and slowly NBS (N-bromosuccinimide, 78.3 g, 0.44 mol) was added as a solid, and the addition was completed after 0.5 h. The reaction system was kept and stirred at 20-25° C. for 24 hours. After the reaction was completed, an aqueous solution of sodium sulfite (500 mL, 0.05 mol / L) was added to the reaction system, the reaction was quenched, and the filter cake was obtained by suction filtration. After washing with deionized water, crystallization was carried out with toluene or absolute ethanol to obtain a white solid. That is intermediate C01-a, the yield is 90.3%.
Embodiment 1-2~ Embodiment 1-14
[0053] Preparation of Example 1-2~Example 1-14 Intermediate A-2~A-14
[0054] The intermediate was prepared according to the method described in Example 1-1, the difference from Example 1-1 was that the N-phenylcarbazole in Example 1-1 was replaced by the reactant in Table 1.
[0055] Table 1 Embodiment 1-1 of the present invention ~Example 1-14 prepares the reaction raw material of intermediate and the structural formula and yield of intermediate
[0056]
[0057]
[0058]
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| luminous efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com