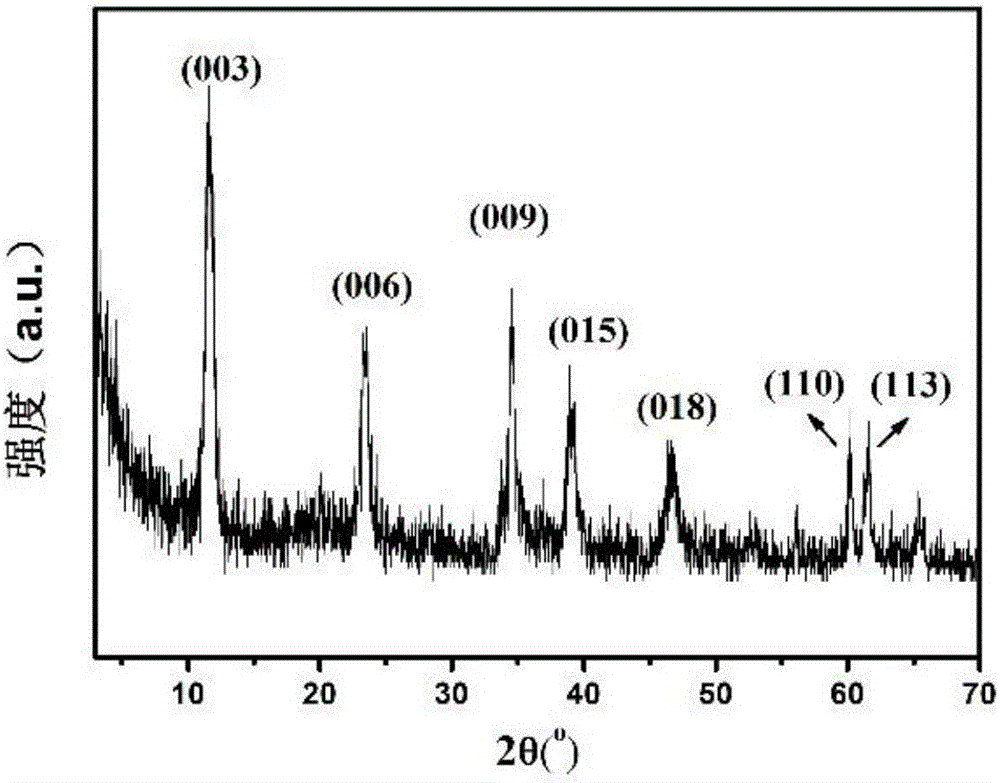
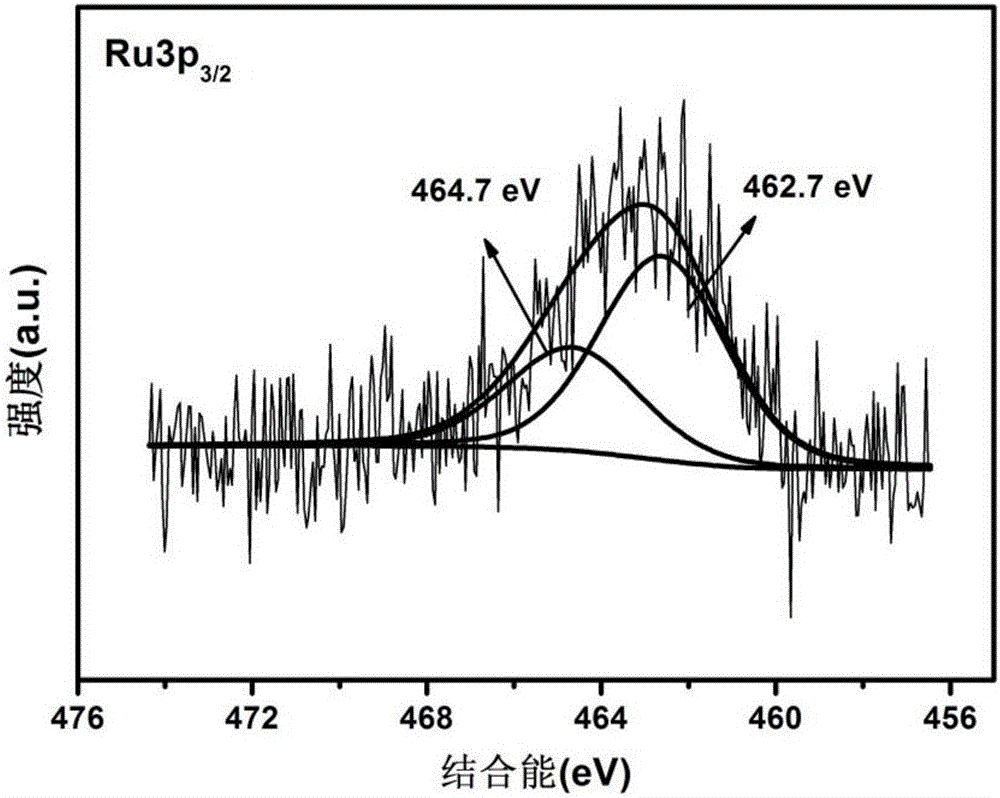
High-dispersion supported ruthenium dioxide catalyst and preparing method thereof
A ruthenium dioxide and catalyst technology is applied in the field of supported precious metal catalysts and their preparation, which can solve the problems of weak interaction between nanoparticles and supports, complex catalyst preparation methods, reduced selectivity and the like, and achieves simple preparation methods and particle size. Small, the effect of improving catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A weighs 1g RuCl 3 ·xH 2 O was dissolved in deionized water to 100mL to prepare RuO 2 The precursor of RuCl 3 solution.
[0032] B with 15mmol Co(NO 3 ) 2 ·6H 2 O and 5mmol Al(NO 3 ) 3 9H 2 O was dissolved in 100 mL deionized water at a molar ratio of 3:1 to prepare a metal salt solution.
[0033] C Add 4.4mL of RuCl prepared in step A to the mixed solution prepared in step B 3 solution, stirred evenly to obtain a mixed solution.
[0034] D 10.4mmol Na 2 CO 3 and 32.3mmol NaOH were added to 100mL deionized water, ultrasonicated until completely dissolved, and prepared as an alkaline solution.
[0035] E. Add the mixed solution prepared in step C and the alkali solution prepared in step D dropwise into the four-neck flask at a constant speed and stir rapidly at room temperature. During the process, the pH value of the solution is always kept at 9-10. After the addition is complete, add The mixed solution was transferred to a water bath at 85°C and stirred f...
Embodiment 2
[0038] Step A is with embodiment 1;
[0039] B with 10mmol Co(NO 3 ) 2 ·6H 2 O and 5mmol Al(NO 3 ) 3 9H 2 O was dissolved in 100 mL deionized water at a molar ratio of 2:1 to prepare a metal salt solution.
[0040] C Add 2.9 mL of RuCl prepared in step A to the mixed solution prepared in step B 3 solution, stirred evenly to obtain a mixed solution.
[0041] D 10.4mmol Na 2 CO 3 and 24.2mmol NaOH were added to 100mL deionized water, and ultrasonicated until completely dissolved to prepare an alkaline solution.
[0042] E. Add the mixed solution prepared in step C and the alkali solution prepared in step D dropwise into the four-neck flask at a constant speed and stir rapidly at room temperature. During the process, the pH value of the solution is always kept at 9-10. After the addition is complete, add The mixed solution was transferred to a water bath at 85°C and stirred for 24 hours; after the stirring was completed, it was lowered to room temperature, and the obtai...
Embodiment 3
[0044] Step A is with embodiment 1;
[0045] B with 15mmol Mg(NO 3 )2 ·6H 2 O and 5mmol Al(NO 3 ) 3 9H 2 O was dissolved in 100 mL deionized water at a molar ratio of 3:1 to prepare a metal salt solution.
[0046] C Add 2.4mL of RuCl prepared in step A to the mixed solution prepared in step B 3 solution, stirred evenly to obtain a mixed solution.
[0047] D 10.4mmol Na 2 CO 3 and 32.3mmol NaOH were added to 100mL deionized water, ultrasonicated until completely dissolved, and prepared as an alkaline solution.
[0048] E. Add the mixed solution prepared in step C and the alkali solution prepared in step D dropwise into the four-neck flask at a constant speed and stir rapidly at room temperature. During the process, the pH value of the solution is always kept at 9-10. After the addition is complete, add The mixed solution was transferred to a water bath at 85°C and stirred for 24 hours; after the stirring was completed, it was lowered to room temperature, and the obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com