Crystalline benzimidazolyl quinoline cuprous complex luminescent material
A technology of benzimidazolyl quinoline and luminescent materials, applied in the field of organic electroluminescent materials and luminescent materials, can solve the problem that the luminous intensity does not meet the application requirements, achieve good phosphorescence emission performance, suppress non-radiative attenuation, equipment simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A large number of phosphorescent complex materials [Cu(2-QBI)(m-Tol 3 P) 2 ]PF 6 Crystal sample preparation: weigh 0.074g (0.2mmol) of Cu(CH 3 EN) 4 PF 6 , 0.122g (0.4mmol) of m-Tol 3 P, 0.048g (0.2mmol) of 2-QBI; after dissolving with 5ml of dichloromethane respectively, mix successively, fully stir to make it fully take place coordination reaction, obtain orange-red clear solution; Add a large amount of n-hexane in the above solution to promote The product crystallized, and a large amount of crystals precipitated after standing for several minutes, which was filtered and vacuum-dried to finally obtain a light orange-yellow crystal product with a yield of 92% (calculated as Cu).
Embodiment 2
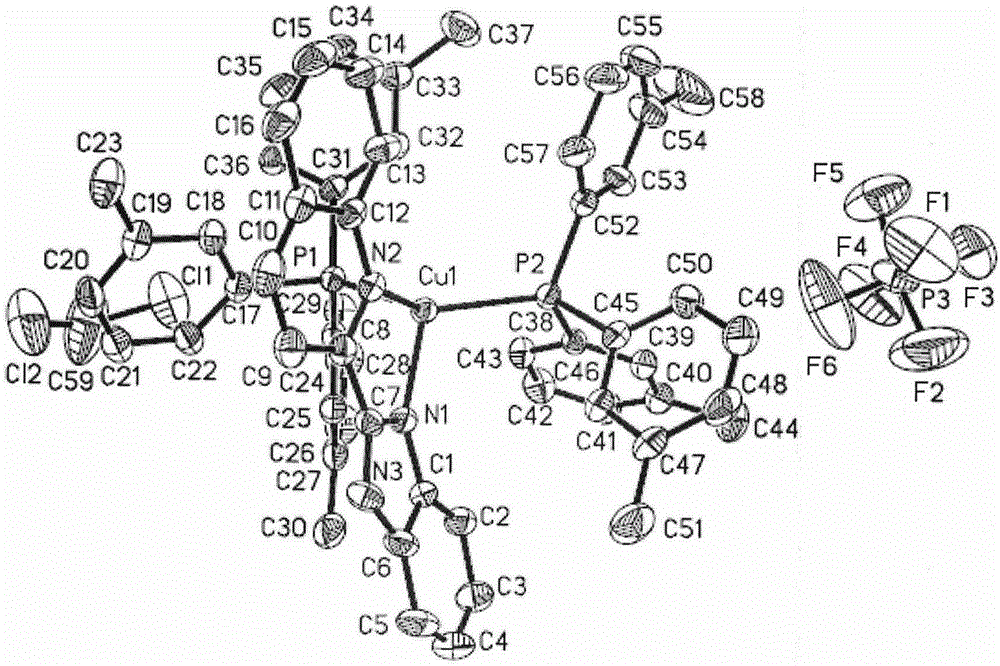
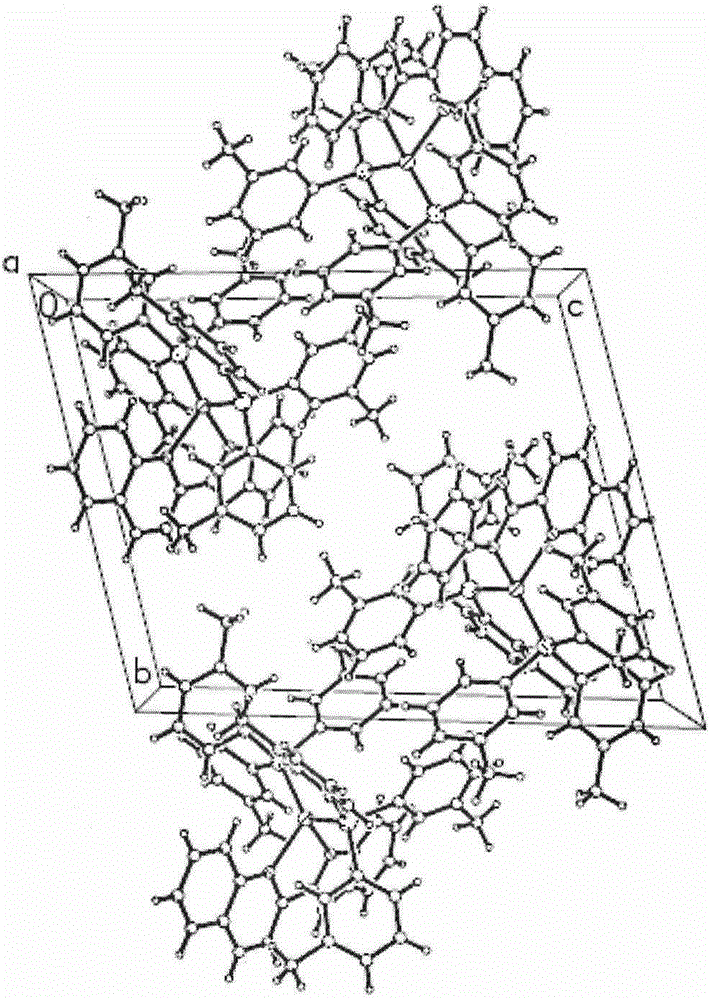
[0031] Synthesis of orange-yellow phosphorescent complex material [Cu(2-QBI)(m-Tol 3 P) 2 ]PF 6 single crystal: Weigh 0.037g (0.1mmol) of Cu(CH 3 EN) 4 PF 6 , 0.061 g (0.2 mmol) of m-Tol 3 P, 0.024g (0.1mmol) of 2-QBI; respectively dissolved in 2ml of dichloromethane and mixed successively, stirred fully to make it fully undergo a coordination reaction to obtain an orange-red clear solution; after filtering, cover the upper layer of the solution with n-hexane The product was promoted to crystallize, and a large number of light orange-yellow massive crystals were precipitated after standing for a few days. A light orange-yellow block crystal with a size of 0.48mm*0.43mm*0.36mm was selected for X-ray single crystal structure test. The molecular structure of the compound is shown in the attached figure 1 , and its unit cell packing structure is shown in the attached figure 2 .
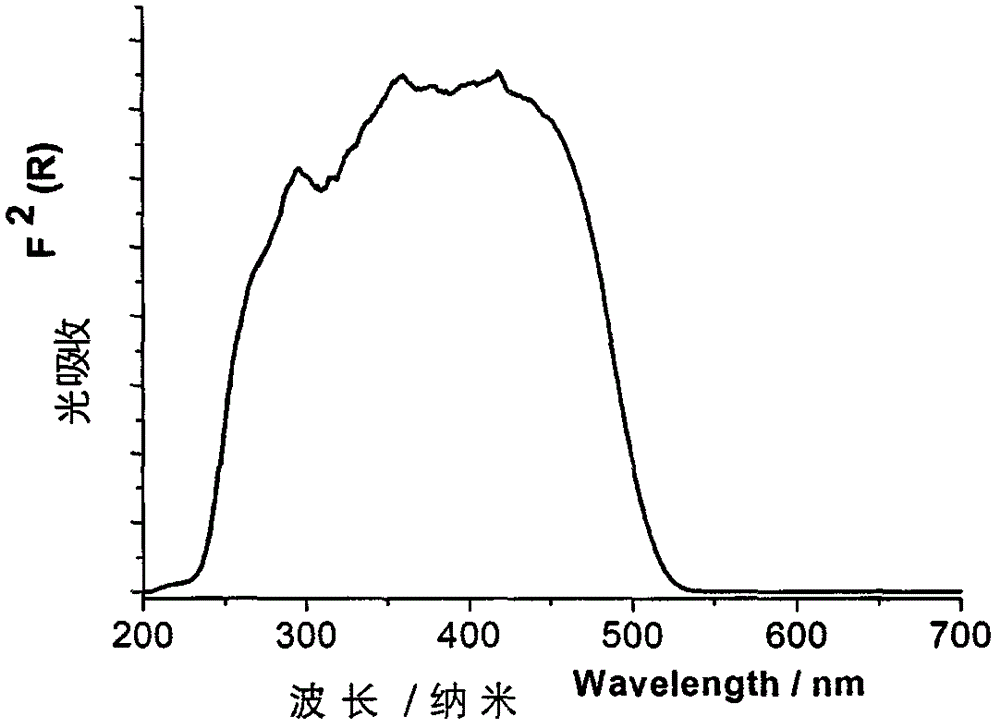
[0032] For the orange-yellow phosphorescent complex material [Cu(2-QBI)(m-Tol 3 P) 2 ]PF ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com