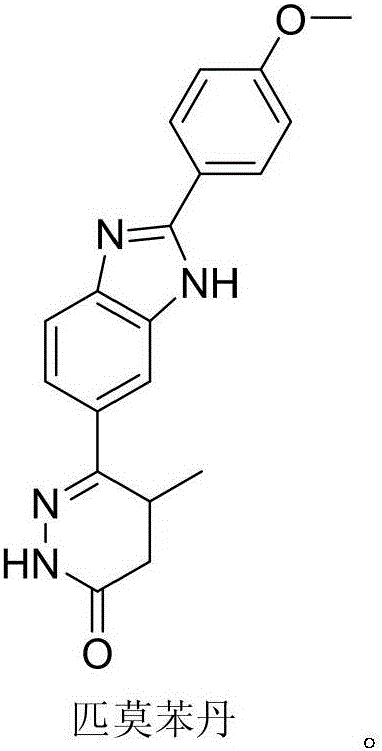
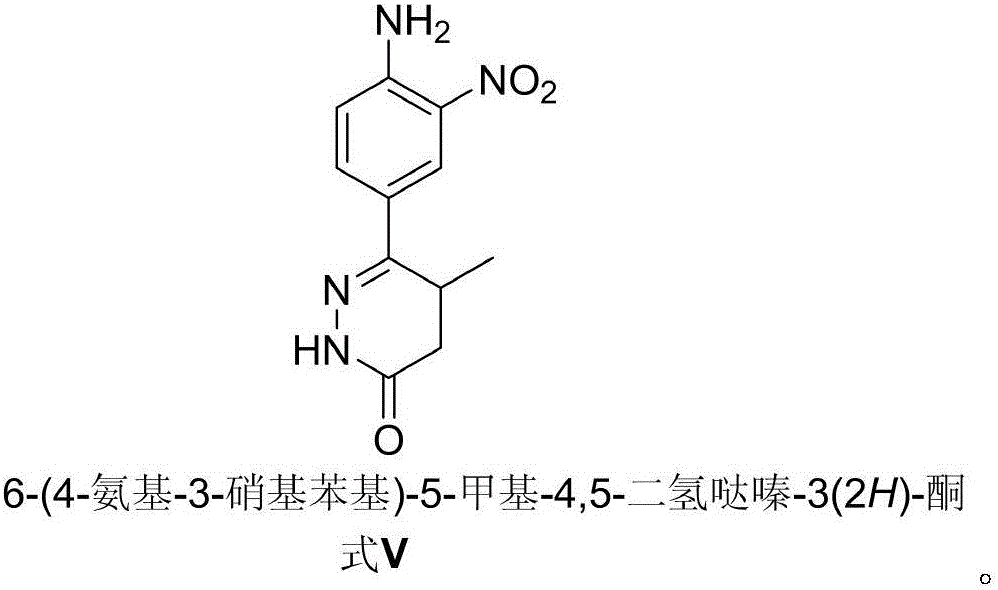
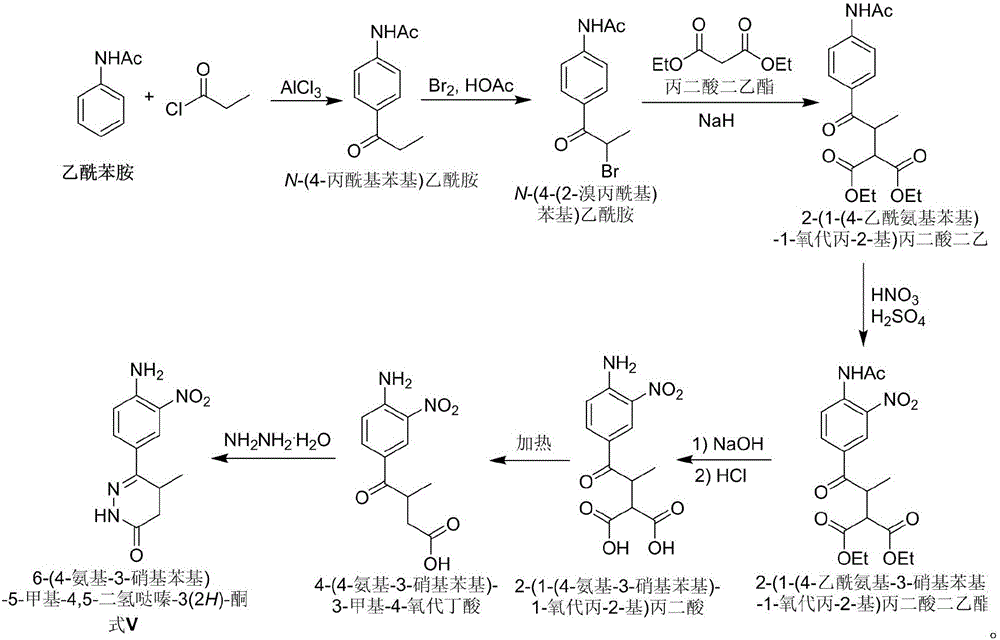
Preparation of pimobendan key intermediate
A technology of nitrophenyl and oxo substitution, which is applied in the direction of organic chemistry and can solve problems that are not conducive to industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0021] 1. Preparation of N-(2-nitro-4-propionylphenyl)acetamide (formula I)
[0022] h 2 SO 4 (200mL) and nitric acid (600mL) were mixed and cooled to below 0°C, then N-(4-propionylphenyl)acetamide (200g, 1.05mol) was added in batches, and the internal temperature of the system was kept below 0 °C for 5 hours. The reaction solution was slowly poured into a mixture of ice water (2000 g) and dichloromethane (2000 mL), stirred for 30 min, and the organic phase was separated after the ice completely turned into water. The aqueous phase was extracted with dichloromethane (800 mL). The organic phases were combined and washed twice with saturated brine (2×2000 mL). After the solvent was removed under reduced pressure, the residue was recrystallized from heptane / ethyl acetate to obtain a pale yellow solid (192 g, 77%).
[0023] 2. Preparation of N-(4-(2-bromopropionyl)-2-nitrophenyl)acetamide (formula II)
[0024] N-(2-nitro-4-propionylphenyl)acetamide (150g, 0.63mol) was added t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com