Application of compounds aszonalenin and acetylaszonalenin in the preparation of antidiabetic drugs
An anti-diabetic, compound technology, applied in the field of natural medicinal chemistry, achieves the effect of inhibiting α-glucosidase, taking a small amount and having a significant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the preparation method of compound 1 and 2
[0020] 1.1 Preparation of Neosartorium fischii ferment by fermentation culture
[0021] 1) Strains: Neosartorium fischii was purchased from the German Biological Resources Collection (DSMZ), and the strain number is DSM3700.
[0022] 2), strain preservation and activation
[0023] Slant strain preparation: 30.0g sucrose, NaNO 3 3.0g, MgSO 4 ·7H 2 O 0.5g, KCl 0.5g, FeSO 4 ·7H 2 O10.0 mg, K 2 HPO 4 1.0g, 13.0g agar, 1000mL distilled water, adjust the pH to 7.2. After high-temperature sterilization, make a slant, insert the purchased bacteria on the solid medium slant, and cultivate at 28°C for 2-5 days. After the end, place it in a refrigerator at 4°C for later use.
[0024] Plate activation: 200g of potatoes, 20g of glucose (or sucrose), 15-20g of agar, 1000mL of tap water, natural pH, and make plates after sterilization. Inoculate the Neosartoria fischii on the slant into the plate, place it at 28° C. ...
Embodiment 2
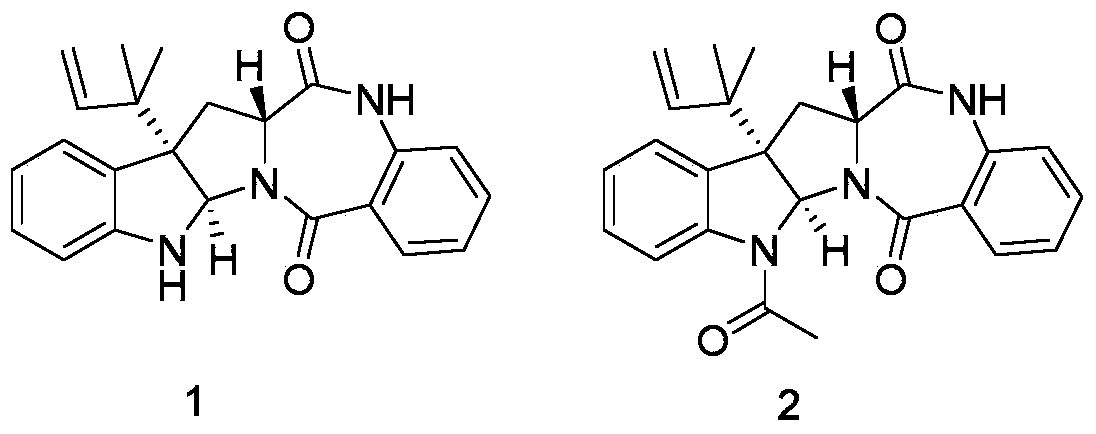
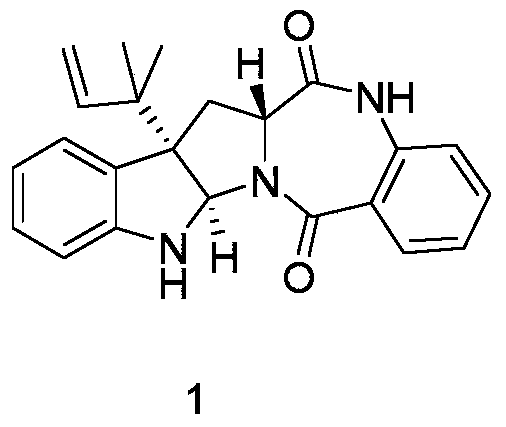
[0031] Example 2: Physicochemical properties and spectral data of compound 1
[0032] Compound 1: colorless transparent crystal; molecular formula is C 23 h 23 N 3 o 2 , optical rotation [α] 20 D =+41.538 (c=0.26, CHCl 3 ),That 1 H-NMR and 13 The C-NMR data are as follows: 1 H-NMR (500MHz, DMSO-d 6 )δ H 10.62(1H,s),7.67(1H,dd,7.9,1.4),7.45–7.54(1H,m),7.19(1H,dd,11.2,4.0),7.13(1H,d,7.4),7.08(1H ,d,7.8),6.95–7.00(1H,m),6.68(1H,s),6.63(2H,dd,16.0,7.9),6.11(1H,dd,17.3,10.8),5.47(1H,s) ,4.99–5.18(2H,m),3.99(1H,dd,8.8,7.4),3.31(1H,dd,13.9,7.2),2.30(1H,dd,13.9,9.1),1.06(3H,s), 0.96(3H,s); 1 H NMR (500MHz, CDCl 3 )δ H7.92(1H,s),7.84(1H,d,7.8),7.45(1H,m),7.22(1H,t,7.6),7.16(1H,d,7.5),7.09(1H,t,7.4), 6.90(1H,d,8.2),6.72(1H,t,7.4),6.62(1H,d,7.9),6.12(1H,dd,17.6,10.6),5.59(1H,s),5.14(1H,d ,10.5),5.11(1H,d,17.6),3.99(1H,t,8.1),3.47(1H,dd,14.2,7.6),2.42(1H,dd,14.0,9.0),1.13(3H,s) ,1.06(3H,s); 13 C-NMR (125MHz, DMSO-d 6 )δ C 169.3, 165.9, 149.2, 144.2, 135.4, 132.3, 13...
Embodiment 3
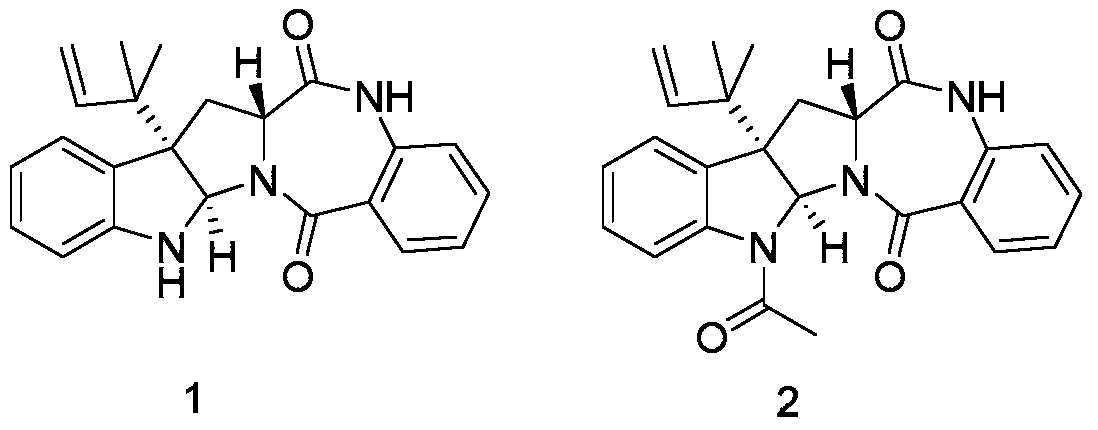
[0035] Example 3: Physicochemical properties and spectral data of compound 2
[0036] Compound 2: Colorless transparent crystal; Molecular formula is C 25 h 25 N 3 o 3 , optical rotation [α] 20 D =-335.71° (c=0.28, MeOH), which 1 H-NMR and 13 The C-NMR data are as follows: 1 H-NMR (500MHz, DMSO-d 6 )δ H 10.68(1H,s),7.81(1H,d,7.9),7.45(2H,m),7.35(1H,d,7.4),7.23(1H,t,7.5),7.09(3H,m),6.06( 1H,s),5.87(1H,dd,17.3,10.8),5.06(2H,m),3.84(1H,t,8.3),3.20(1H,dd,13.7,8.3),2.47(3H,s), 2.42(1H,dd,13.7,8.4),1.25(1H,d,12.2),1.10(3H,s),0.92(3H,s); 1 H NMR (500MHz, CDCl 3 )δ H 8.02(1H,d,7.9),7.72(1H,d,7.8),7.64(1H,s),7.41(1H,t,7.5),7.28(1H,t,7.6),7.24(1H,d,7.6 ),7.19(1H,t,7.6),7.07(1H,t,7.5),6.86(1H,d,8.2),5.93(1H,s),5.89(1H,dd,17.5,10.4),5.15(1H ,d,10.4),5.12(1H,d,17.5),3.90(t,8.4),3.37(1H,dd,13.9,8.5),2.59(3H,s),2.45(1H,dd,13.9,8.2) ,1.20(3H,s),0.99(3H,s); 13 C NMR (125MHz, DMSO-d 6 ) δ C 169.7,168.7,165.9,143.7,141.6,135.1,134.1,132.1,130.0,128.3,126.9,125.1,124.2,123.6,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com