Application of isoproterenol and its pharmaceutically acceptable salts in the preparation of antitumor drugs
A technology of isoproterenol and anti-tumor drug, applied in the field of anti-tumor drugs, can solve the problem that there is no active ingredient of isoproterenol, and achieve the effects of clear toxicology and pharmacology, high promotion and application value, and low research and development cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The present invention will be further described below in conjunction with embodiment.
[0022] Experimental Materials
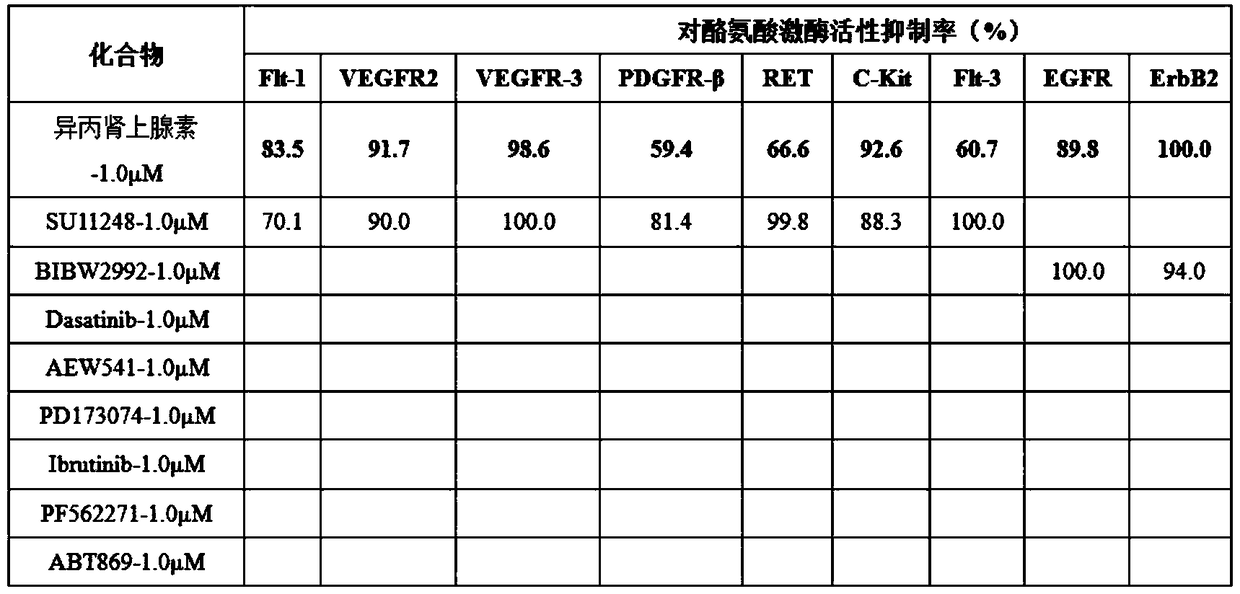
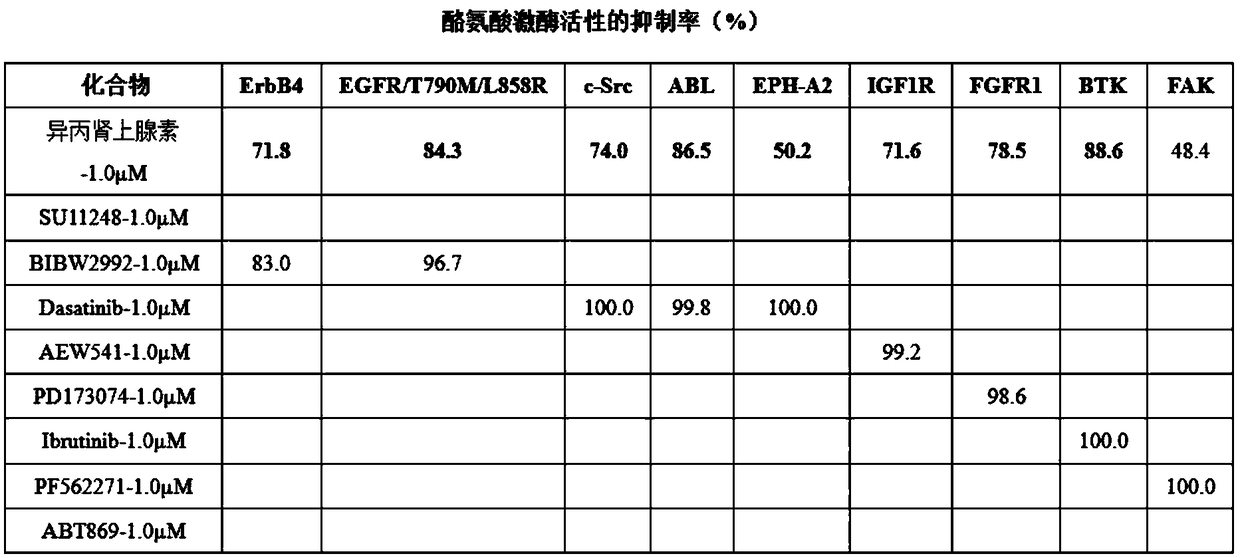
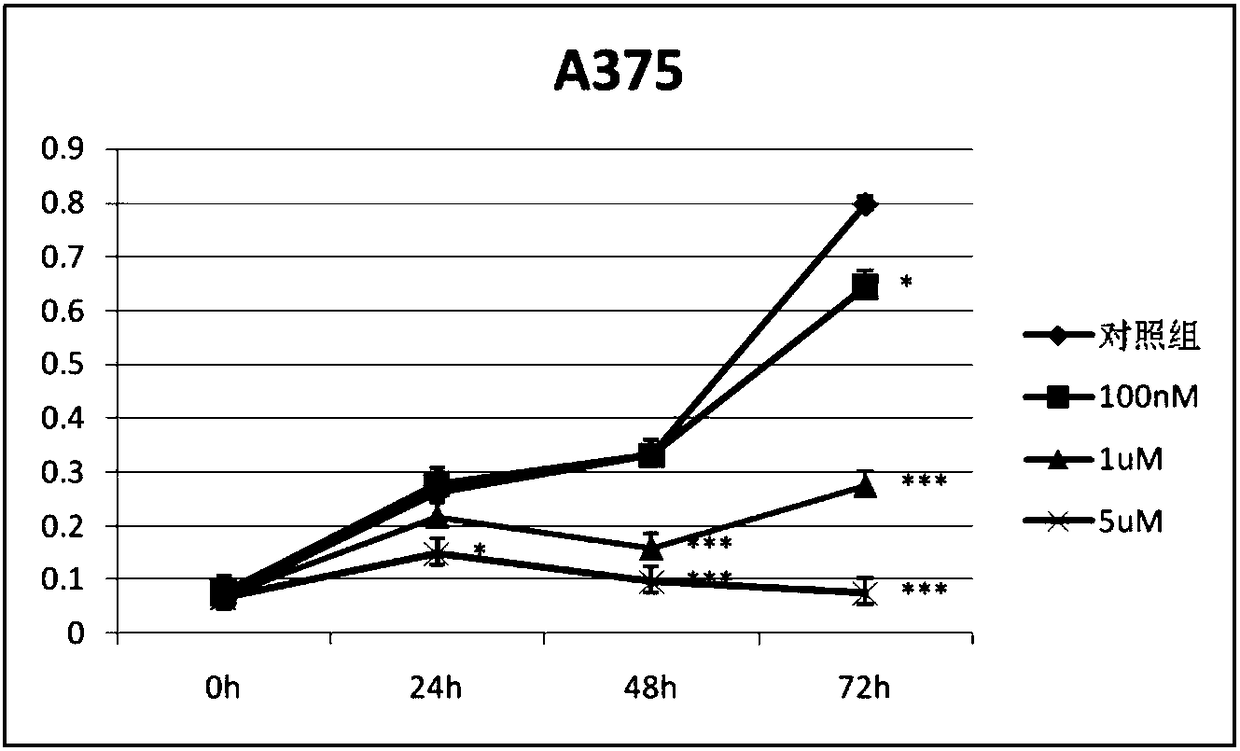
[0023] Cell lines: Human malignant melanoma A375, SK-mel-28, SK-mel-5, B16F10 cell lines, lung cancer A549 cell line, colon cancer HCT116 cell line, and black drug-resistant strain RA are all preserved in our laboratory . Isoproterenol (No. I129810, purity ≥ 99%) was purchased from Shanghai Aladdin Biotechnology Co., Ltd. The mother solution was dissolved in PBS to the corresponding concentration and stored at -20°C. High-glucose DMEM medium, fetal bovine serum, and trypsin were all purchased from Hyclone Company of the United States, and Matrigel was purchased from BD Company of the United States. CO 2 The incubator was purchased from Thermo Company of the United States, the inverted microscope was purchased from Olympus Company of Japan, and the desktop centrifuge was purchased from BECKMAN Company of the United States.
[0024] experimental met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com