Breast cancer diagnosis kit based on combined detection of FGF1, FGF10 and IL6
A diagnostic kit and breast cancer technology, applied in the field of protein detection, to achieve the effects of low cost, good accuracy, and widening the optional space
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1. Selection and verification of the markers described in the present invention
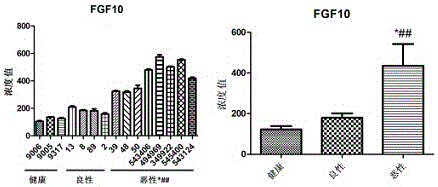
[0039] The detection of FGF10 used the ELISA kit of Uscn Company. from figure 1 We can clearly see the differences between the three groups of samples. The serum FGF10 concentration of healthy people is 104-135pg / ml, the concentration of benign tumors is between 158-208pg / ml, and the serum concentration of malignant tumors is 158-208pg / ml. The concentration is 318-575pg / ml. Statistical analysis showed that benign vs malignant P=0.004<0.05, malignant vs healthy P=0.0121<0.05 were significantly different.
Embodiment 2
[0040] Embodiment 2, kit of the present invention and preparation thereof
[0041] This embodiment provides a diagnostic kit for breast cancer, which includes a reagent for quantitative detection of fibroblast growth factor-10.
[0042] Specifically, the agent is an antibody to fibroblast growth factor 10.
[0043] Further, the diagnostic kit for breast cancer also includes conventional reagents used in enzyme-linked immunosorbent assay.
[0044] This embodiment also provides a preparation method of a breast diagnostic kit, comprising the following steps: assembling an antibody for fibroblast growth factor 10 into the kit; and / or
[0045] Antibody to fibroblast growth factor 10 is placed in the package marked breast cancer diagnostic use.
[0046] The steps further include: the reagents for assembling the kit also include conventional reagents used in ELISA; and / or
[0047] The conventional reagents used in the enzyme-linked immunosorbent assay are also placed in the packag...
Embodiment 3
[0050] Embodiment 3, adopt kit described in the present invention to carry out breast cancer diagnosis
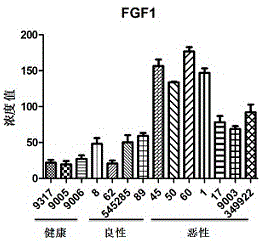
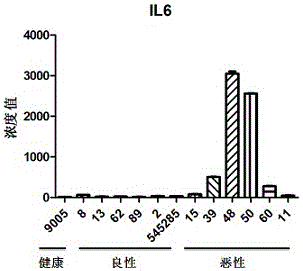
[0051] Such as Figure 1-Figure 3 As shown, by detecting the concentrations of IL6, IL9, IL21, FGF1, FGF10, and FGF14 in the serum samples of healthy, benign, and malignant groups, we finally selected a group that can be detected in most of the samples and is detected in malignant patients and healthy controls. IL6, FGF1, and FGF10, which had significant differences between groups, were used as markers for the diagnosis of breast cancer. In addition, we also detected the now-recognized tumor marker CEA in serum as a positive control. The data showed that in samples No. 1, 545400, No. 50, and No. 48 of which CEA was determined to be breast cancer, FGF1 , FGF10 or IL6 concentration is also quite high. And in samples No. 39, No. 45, No. 494969, and No. 349922 whose CEA concentration was within the normal range, one or both of IL6, FGF1, and FGF10 were also higher than normal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com