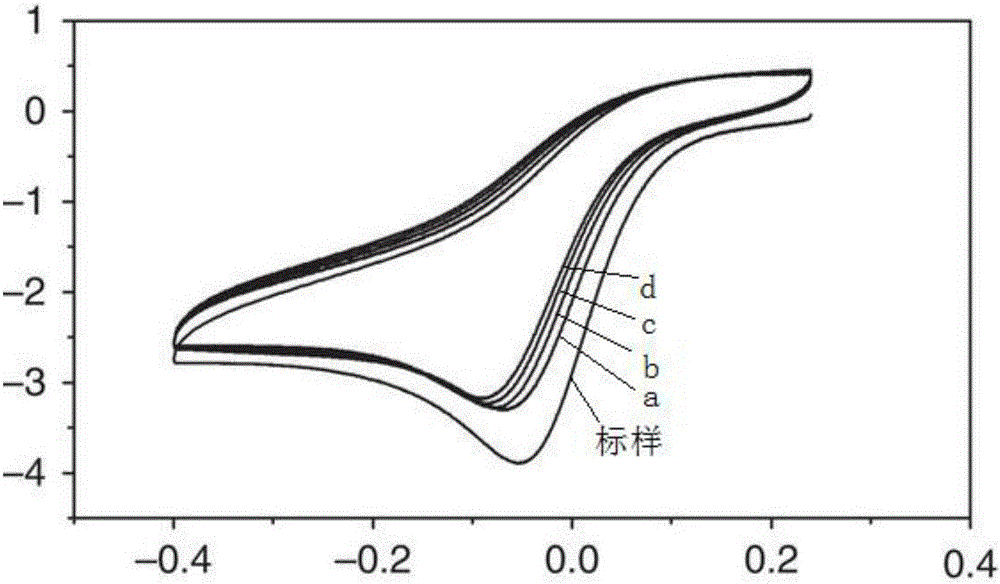
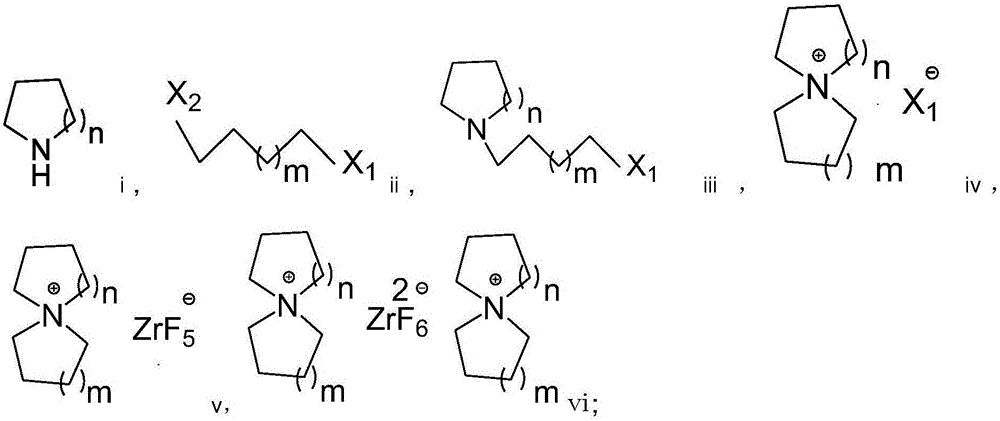
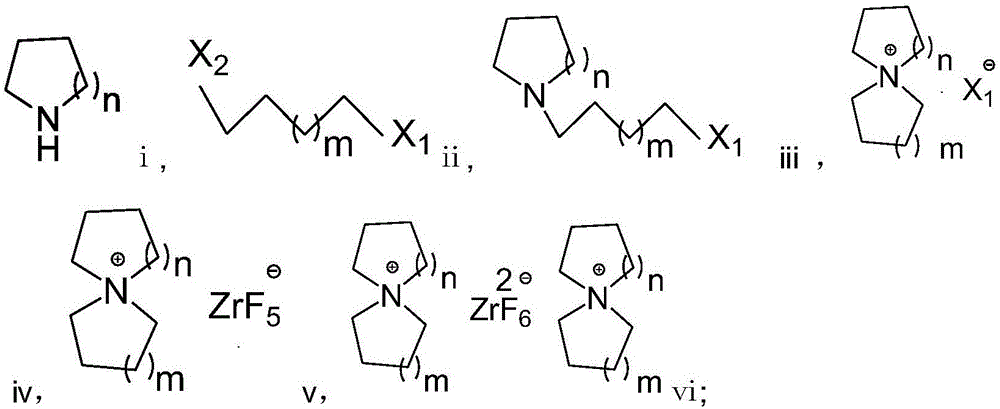
Preparation method of fluorozirconate
A technology of fluorozirconate and pentafluorozirconate, which is applied in electrical components, electrolytic capacitors, capacitors, etc., can solve the problems of unachieved, and achieve the effects of high yield, easy purification and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 A kind of preparation method of fluorozirconate
[0025] 1) Weigh 7.1g of pyrrolidine and 14g of potassium carbonate, dissolve it in 1000mL of acetone, add it to a three-neck flask, adjust the temperature to 15-20°C, and slowly add 10mL of 1-bromo-3-chloropropane dropwise for 5 minutes , the temperature of the whole dropwise addition reaction was kept at 15-20°C. After the dropwise addition was completed, the temperature was raised to 35°C, and the reaction was continued for 24 hours. After the reaction was completed, the reaction solution was directly filtered, and the organic solvent was distilled off under reduced pressure to obtain 13.8g 1-(3-chloro Propane) pyrrole, the yield (calculated as pyrrolidine) is 94%.
[0026] 2) Weigh 13.8g of 1-(3-chloropropane)pyrrolidine and dissolve it in 850mL of acetonitrile, then add it into a three-neck flask, the temperature rises to 60-65°C, the whole reaction temperature is kept at 60°C-65°C, and the reaction is c...
Embodiment 2
[0029] 1) Weigh 7.1g of pyrrolidine and 14g of potassium carbonate, dissolve it in 1000mL of acetone, add it to a three-neck flask, adjust the temperature to 15°C, and slowly add 13.4mL of 1,4-diiodobutane dropwise. At 15°C to 20°C, after the addition, the temperature was raised to 35°C, and the reaction was continued for 24 hours. After the reaction was completed, the reaction solution was directly filtered, and the organic solvent was distilled under reduced pressure to obtain 24.5g of 1-(4-iodobutane)pyrrole. The yield ( Based on pyrrolidine) was 97%.
[0030] 2) Weigh 24.5g of 1-(4-iodobutane)pyrrole and dissolve it in 850mL of acetonitrile, add it into a three-neck flask, raise the temperature to 60°C, keep the whole reaction temperature at 60°C-65°C, and continue the reaction for 16 hours. After the reaction is completed, the temperature is down to room temperature, and the reaction solution is directly filtered, and the organic solvent is distilled under reduced pressur...
Embodiment 3
[0033] 1) Weigh 7.1g of pyrrolidine and 10.8g of sodium carbonate and dissolve them in 1000mL of acetone, add them to a three-neck flask, adjust the temperature to 15°C and slowly add 13.8mL of 1,5-dibromopentane, and keep the entire reaction temperature at 15°C ℃~20℃, after the addition is completed, the temperature is raised to 35℃, and the reaction is continued for 24h. After the reaction is completed, the reaction solution is directly filtered, and the organic solvent is distilled under reduced pressure to obtain 21.5g of 1-(5-bromopentane)pyrrole. The yield (calculated as pyrrolidine Count) is 98%.
[0034] 2) Weigh 24.5g of 1-(5-bromopentane)pyrrole and dissolve it in 850mL of acetonitrile, add it into a three-neck flask, raise the temperature to 60°C, keep the whole reaction temperature at 60°C~65°C, continue the reaction for 16h, wait After completion of the reaction, the temperature is down to room temperature, and the reaction solution is directly filtered, and the o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com