A kind of method of phenolic compound catalytic oxidation synthesis benzoquinone compound
A phenolic compound, catalytic oxidation technology, applied in the preparation of oxidized quinone, organic chemistry, etc., to achieve the effects of environmental friendliness, mild reaction conditions, and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
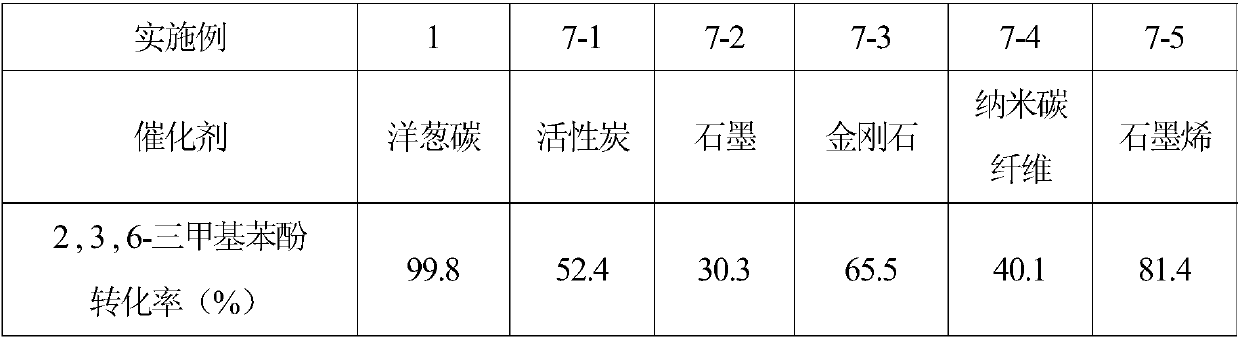
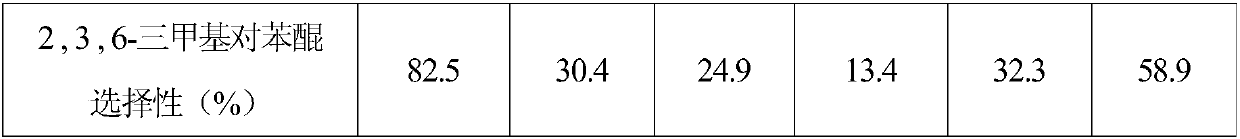
Embodiment 1
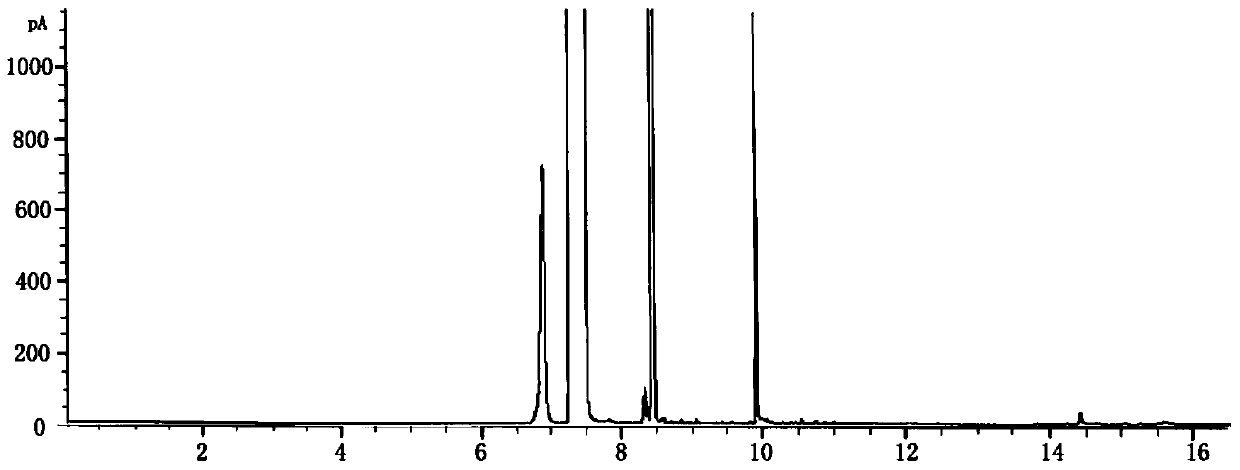
[0025] Add 0.1 mmol of 2,3,6-trimethylphenol, benzotrifluoride, solid catalyst onion carbon, and oxidant tert-butyl hydroperoxide into a closed glass container for mixing, and then ultrasonicate to form a suspension (5 min). Among them, benzotrifluoride is 5ml, and the molar ratio of onion carbon to 2,3,6-trimethyl-p-phenol is 6.66:1. The molar ratio of oxidizing agent to 2,3,6-trimethyl-p-phenol is 3.6:1. The mixed suspension was placed in an oil bath and heated to 80 °C under stirring. After reacting for 12 hours, take out reaction vessel from oil bath, cool to room temperature, pour out liquid-solid mixture, it is filtered, obtain solid catalyst and remaining liquid phase mixture, add a certain amount of internal standard (ethylbenzene), to This liquid phase mixture is analyzed, and the retention time ( figure 1 ) are: tert-butanol 6.9min, trifluorotoluene 7.4min, 2,3,6-trimethyl-p-benzoquinone 9.91min, 2,3,6-trimethylphenol 9.98min, ethylbenzene 8.4min. The selectivity ...
Embodiment 2
[0027] Add 0.1 mmol of 2,3,6-trimethylphenol, benzotrifluoride, solid catalyst onion carbon, and oxidant tert-butyl hydroperoxide into a closed glass container for mixing, and then ultrasonicate to form a suspension (5 min). Among them, benzotrifluoride is 5ml, and the molar ratio of onion carbon to 2,3,6-trimethylphenol is 6.66:1. The molar ratio of oxidizing agent to 2,3,6-trimethylphenol is 3.6:1. The mixed suspension was placed in an oil bath and heated to the temperature shown in Table 1 under stirring conditions. After reacting for 12 hours, take out reaction vessel from oil bath, cool to room temperature, pour out liquid-solid mixture, it is filtered, obtain solid catalyst and remaining liquid phase mixture, add a certain amount of internal standard (ethylbenzene), to The liquid phase mixture is analyzed, and the conversion rate of 2,3,6-trimethylphenol and the selectivity results of 2,3,6-trimethyl-p-benzoquinone are shown in Table 1. It can be seen that the temperatu...
Embodiment 3
[0032]Add 0.1 mmol of 2,3,6-trimethylphenol, trifluorotoluene, solid catalyst onion carbon, and oxidant tert-butyl hydroperoxide into a closed glass container and mix them, and form a suspension under ultrasonic treatment (5 minutes). Among them, benzotrifluoride is 5ml, and the molar ratio of onion carbon to 2,3,6-trimethylphenol is 6.66:1. The molar ratio of oxidizing agent to 2,3,6-trimethylphenol is 3.6:1. The mixed suspension was placed in an oil bath and heated to 80 °C under stirring. Reaction time to Table 2, take out reaction vessel from oil bath, cool to room temperature, pour out liquid-solid mixture, it is filtered, obtain solid catalyst and remaining liquid phase mixture, add a certain amount of internal standard (ethylbenzene), The liquid phase mixture is analyzed, and the conversion rate of 2,3,6-trimethylphenol and the selectivity of 2,3,6-trimethyl-p-benzoquinone are measured. The results are shown in Table 2. It can be seen that prolonging the time is benefi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com