Sulfo-imidazole-diketone and imidazole-diketone compound and applications thereof
A kind of technology of compound and solvate, applied in the field of thioimidazole dione
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
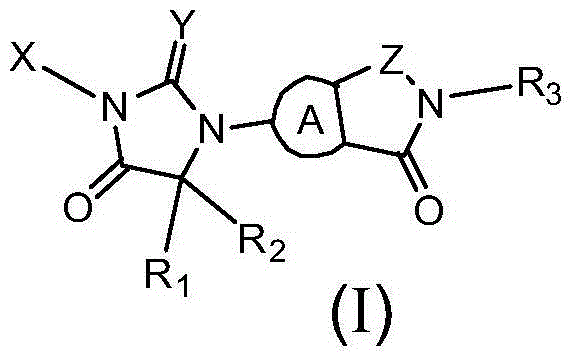
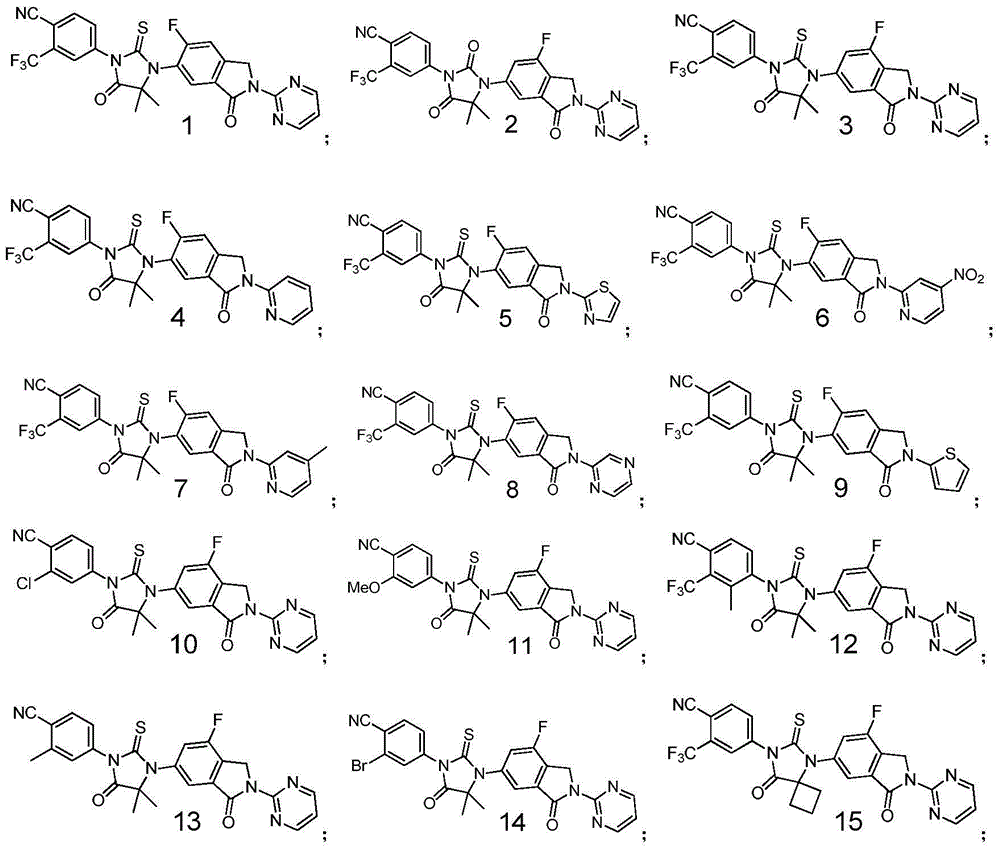
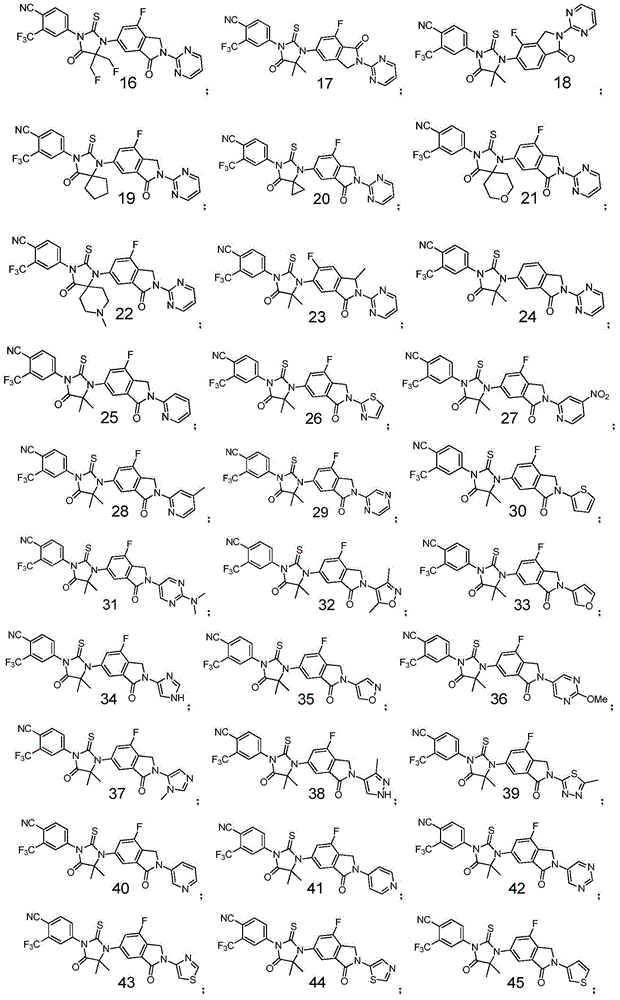
[0092] 4-[3-(6-fluoro-3-oxo-2-(pyrimidin-2-yl)isoindolin-5-yl)-4,4-dimethyl-5-oxo-2- Thioimidazolidin-1-yl]-2-trifluoromethylbenzonitrile
[0093] Step 1: Preparation of 4-isothiocyanato-2-trifluoromethylbenzonitrile
[0094]
[0095] Thiophosgene (1.37ml, 17.97mmol) was added to water (22ml), stirred for 8 minutes, and then 4-amino-2-trifluoromethylbenzonitrile (2.23g, 11.98mmol) was slowly added to the above After the addition, the mixture was stirred at room temperature for 1.5h. The completion of the reaction was monitored by thin-layer chromatography, extracted with dichloromethane, the organic phases were combined, washed with saturated brine, filtered, and concentrated under reduced pressure to obtain 2.73 g of light yellow oily liquid with a yield of 99%. 1 H NMR (400MHz, CDCl 3 ): δ=7.86 (d, J=8.3Hz, 1H), 7.61 (d, J=1.4Hz, 1H), 7.51 (dd, J=8.3, 1.7Hz, 1H).
[0096] Step 2: Preparation of 2-bromo-4-fluoro-5-nitrobenzoic acid
[0097]
[0098] Under ice bath,...
Embodiment 2
[0121] 4-[3-(7-fluoro-3-oxo-2-(pyrimidin-2-yl)isoindolin-5-yl)-4,4-dimethyl-2,5-dioxo imidazolidin-1-yl]-2-trifluoromethylbenzonitrile
[0122]
[0123] Compound 2 is obtained by referring to the synthesis method of scheme 1 and scheme 2 for the compound of the present invention. ESI-MS m / z:524.8(M+H) + .
Embodiment 3
[0125] 4-[3-(7-fluoro-3-oxo-2-(pyrimidin-2-yl)isoindolin-5-yl)-4,4-dimethyl-5-oxo-2- Thioimidazolidin-1-yl]-2-trifluoromethylbenzonitrile
[0126]
[0127] The compound of the present invention was synthesized with reference to the synthesis method of Example 1 to obtain compound 3. ESI-MS m / z:540.7(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com