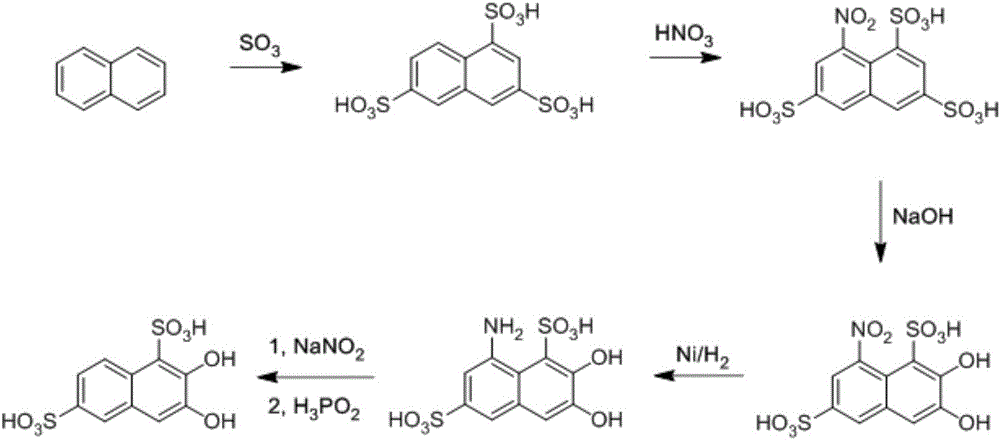
Synthesis method of 2, 3-dihydroxynaphthalene-1, 6-disulfonic acid chemical intermediate
A dihydroxynaphthalene and synthesis method technology, applied in the preparation of sulfonic acid, organic chemistry, etc., can solve the problems of low product selectivity, difficult industrial production, and difficult availability of raw materials, and achieve high product selectivity, simple operation, and high selectivity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 100g of 8-nitro-1,3,6-naphthalenetrisulfonic acid solid and 116g of 10% sodium hydroxide solution into a 500mL four-neck flask, stir thoroughly for 0.5 hours, heat up to 90°C, and keep warm at this temperature After reacting for 1 hour, the temperature was then lowered to 25°C, and a large amount of solids precipitated out of the system, which was filtered and dried to obtain 83.4 g of 2,3-hydroxy-8-nitro-1,6-naphthalene disulfonic acid, with an analytical content of 99.1%. Yield 95.1%.
Embodiment 2-4
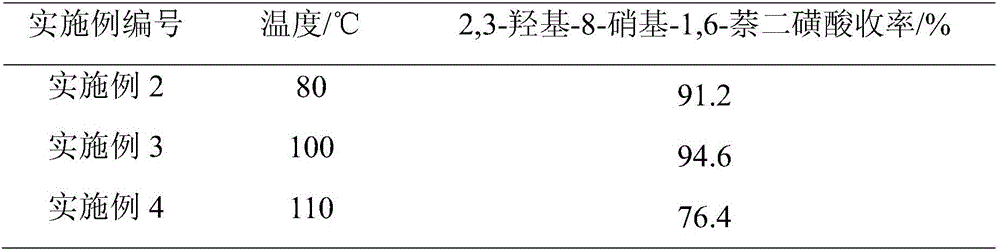
[0024] On the basis of Example 1, different reaction temperatures were adjusted, and other conditions were unchanged. The obtained results are shown in Table 1.
[0025] The influence of table 1 different reaction temperature on product yield
[0026]
Embodiment 5-6
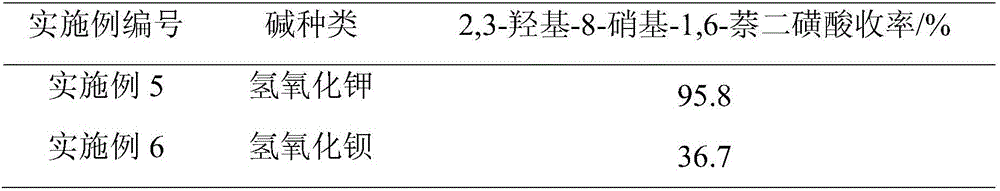
[0028] On the basis of Example 1, different types of bases were investigated, and other conditions remained unchanged. The obtained results are shown in Table 2.
[0029] The impact of the different types of alkalis on the product yield in table 2
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com