Antimicrobial peptide Lexapeptide, and preparation method and application thereof
A technology of antimicrobial peptides and peptides, applied in biochemical equipment and methods, antibacterial drugs, antifungal agents, etc., can solve the problems of narrow antibacterial spectrum and insufficient stress resistance, and achieve good tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The present invention relates to a novel high-efficiency lanthipeptide antibacterial peptide Lexapeptide and its preparation method and application; wherein,
[0054] LB medium: used for the cultivation of Escherichia coli;
[0055] Tryptone 10g, yeast extract 5g, sodium chloride 5g, deionized water to 1,000ml, pH 7.0.
[0056] 2×YT medium: Escherichia coli-Streptomyces indirect conjugative transfer;
[0057] Tryptone 16g, yeast extract 10g, sodium chloride 5g, deionized water to 1,000ml, pH 7.0.
[0058] Mueller Hinton medium: used for culturing Staphylococcus aureus, Staphylococcus epidermidis, etc. during MIC determination, purchased from Oxoid.
[0059] SFM medium: Streptomyces culture sporulation and Escherichia coli-Streptomyces indirect conjugative transfer;
[0060] Soybean cake powder 20g, mannitol 20g, tap water to 1,000ml, pH 7.2-7.4.
[0061] Soak 20 g of soybean cake powder in 1,000 ml of tap water, sterilize at 121°C for 20 minutes, and then filter and...
Embodiment 1
[0072] Example 1, Heterologous Expression of Bacterial Artificial Chromosome Containing Lexapeptide Biosynthetic Gene Cluster
[0073] (1) The bacterial artificial chromosome library (8 pieces of 96-well plates) of Streptomyces rochei Sal35 was taken out from the -80°C refrigerator, and placed in a 37°C incubator for 2 hours until it melted completely.
[0074] (2) Add 130 μl of fresh LB medium to a 96-well plate, use a replicator to inoculate the bacterial artificial chromosome library of Streptomyces rochei Sal35 into a 96-well plate, and incubate at 37°C and 220 rpm for 4 to 6 hours .
[0075] (3) At the same time, inoculate the ET12567 / pUB307 strain in a 250ml Erlenmeyer flask containing 100ml of LB medium containing 50μg / m1 kanamycin, and culture it at 37°C and 220rpm for 4-6 hours.
[0076] (4) Collect ET12567 / pUB307 cells by centrifugation at 4,000rpm for 10 minutes, wash with fresh LB medium for 3 times, and then resuspend with 15ml of fresh LB.
[0077] (5) Take 1...
Embodiment 2
[0083] Embodiment 2, the detection of Lexapeptide
[0084] (1) The 6A8 clone was selected from the bacterial artificial chromosome library of Streptomyces rochei Sal35 and cultured. Then, the bacterial artificial chromosome was introduced into the heterologous expression host Streptomyces lividans SBT5 by Escherichia coli-Streptomyces indirect conjugative transfer to obtain a Lexapeptide-producing strain Streptomyces lividans SBT5 / Sal35 6A8 CGMCC No.12751.
[0085] (2) Inoculate the Lexapeptide-producing bacterial artificial chromosome heterologous expression strain on solid YBP medium, ferment 3 plates for each strain, and ferment and culture at 30° C. for 4 to 6 days.
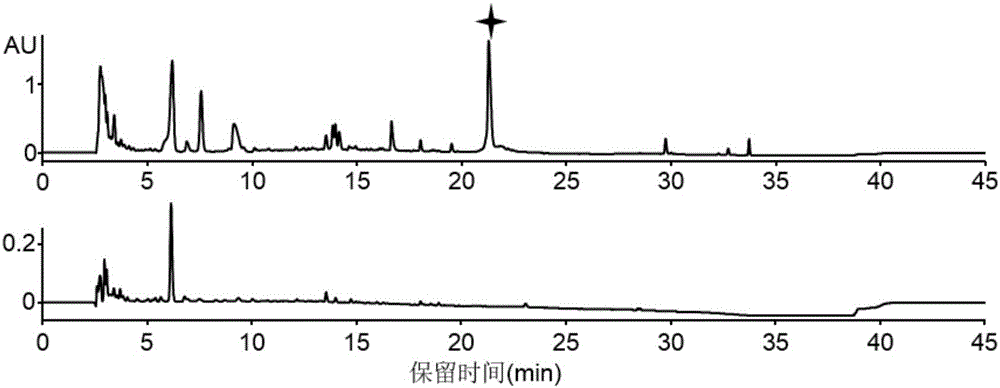
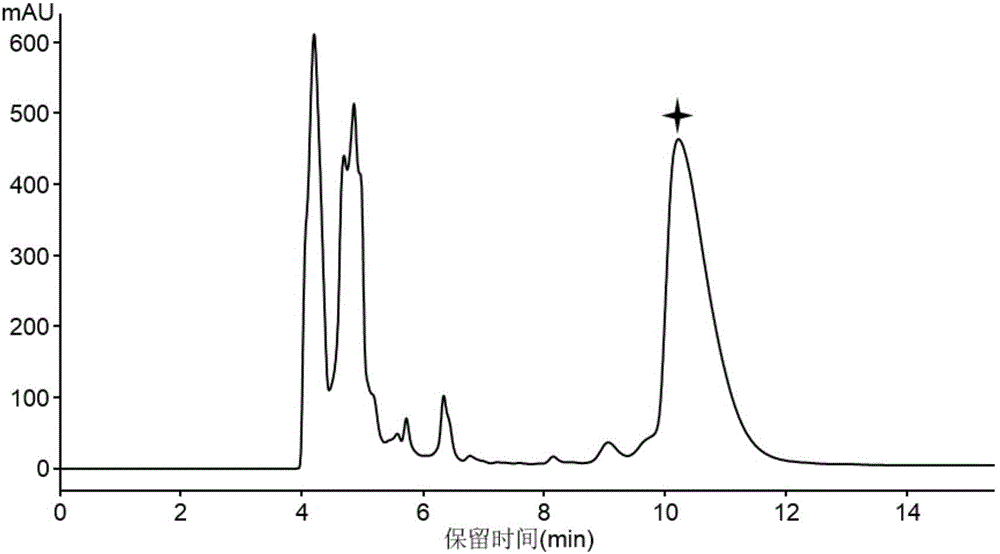
[0086] (3) After the fermentation, the solid fermented product was collected, extracted three times with an equal volume of methanol, concentrated in vacuo and spin-dried to obtain crude methanol extract.
[0087] (4) The crude methanol extract was dissolved in 10 ml of sterile water, extracted three times...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com