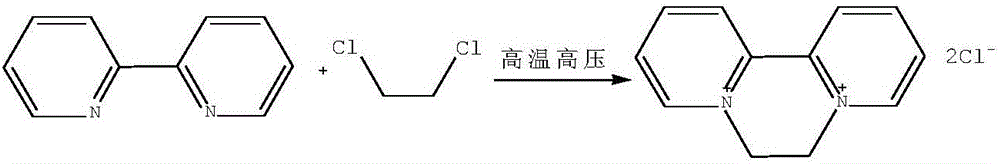
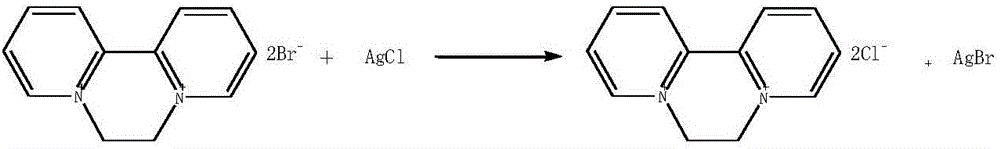
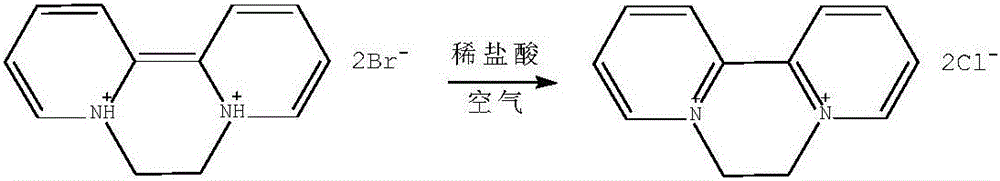
Preparation method of aquacide dichloride
A technology for diquat and chloride salts, applied in the field of preparation of diquat dichloride salts, can solve the problems of unobtainable raw materials, low product purity and high cost, and achieves overcoming high production costs, obvious economic benefits, and low production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In a 2000ml four-neck flask equipped with a mechanical stirrer, a thermometer and a distillation device, add 640g (1.86mol) diquat dibromide salt, add water to prepare a diquat dibromide salt solution with a mass concentration of 40%, and start stirring , raise the temperature to 80°C, adjust the chlorine gas feed rate, and maintain the reaction temperature at 85°C for the reaction. The bromine produced during the reaction is captured by the condensation device, and the tail gas is absorbed by adding lye. When the reaction temperature dropped to 80°C, the reaction was over, the chlorine flow was stopped, and the residual bromine and chlorine gas in the solution were purged with air, detected by KI-starch test paper, and 185 g (2.6 mol) of chlorine gas was consumed in total for the electronic scale metering reaction to obtain the product Diquat dichloride 470g (1.85mol), molar yield 99.5%, the captured crude bromine was dewatered and dried with phosphorus pentoxide to fin...
Embodiment 2
[0025] In a 2000ml four-neck flask equipped with a mechanical stirrer, a thermometer and a reflux device, add 640g (1.86mol) diquat dibromide salt, add water to prepare a diquat dibromide salt solution with a mass concentration of 40%, and start stirring , the temperature was raised to 45°C, and the chlorine gas feeding rate was adjusted to maintain the reaction temperature at 50°C. When the reaction temperature dropped to 45°C, the reaction was over, and the bromine was distilled and separated by heating. After the distillation is finished, the residual bromine and chlorine in the solution are flushed with nitrogen, and detected by KI-starch test paper. Electronic weighing reaction consumes 156.2g (2.2mol) of chlorine gas in total, and obtains 468.6g (1.84mol) of diquat dichloride, a molar yield of 99.1%, and the collected crude bromine is dried by removing water and phosphorus pentoxide , finally got bromine 286.8g, bromine yield 96.3%.
Embodiment 3
[0027] In a 2000ml four-necked flask equipped with a mechanical stirrer, a thermometer and a distillation device, add 320g (0.93mol) diquat dibromide salt, add water to prepare a diquat dibromide salt solution with a mass concentration of 20%, and start stirring , raise the temperature to 80°C, adjust the chlorine gas feed rate, and maintain the reaction temperature at 83°C for the reaction. The bromine produced during the reaction is captured by the condensation device, and the tail gas is absorbed by adding lye. When the reaction temperature dropped to 80°C, the reaction was over, the chlorine flow was stopped, and the residual bromine and chlorine in the solution were purged by air, and detected by KI-starch test paper. The metering reaction of the electronic scale consumes 79g (1.1mol) of chlorine gas in total, and 235.7g (0.928mol) of the product diquat dichloride is obtained, and the molar yield is 99.8%. Finally, 143.4 g of bromine was obtained, and the yield of bromine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com