Cancer immunotherapy by delivering class II MHC antigens using a VLP-replicon
A technology of replicators and antigens, which is applied in the direction of cancer antigen components, antibody medical components, virus antigen components, etc., and can solve problems such as easy contamination, trouble, and excessive length
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
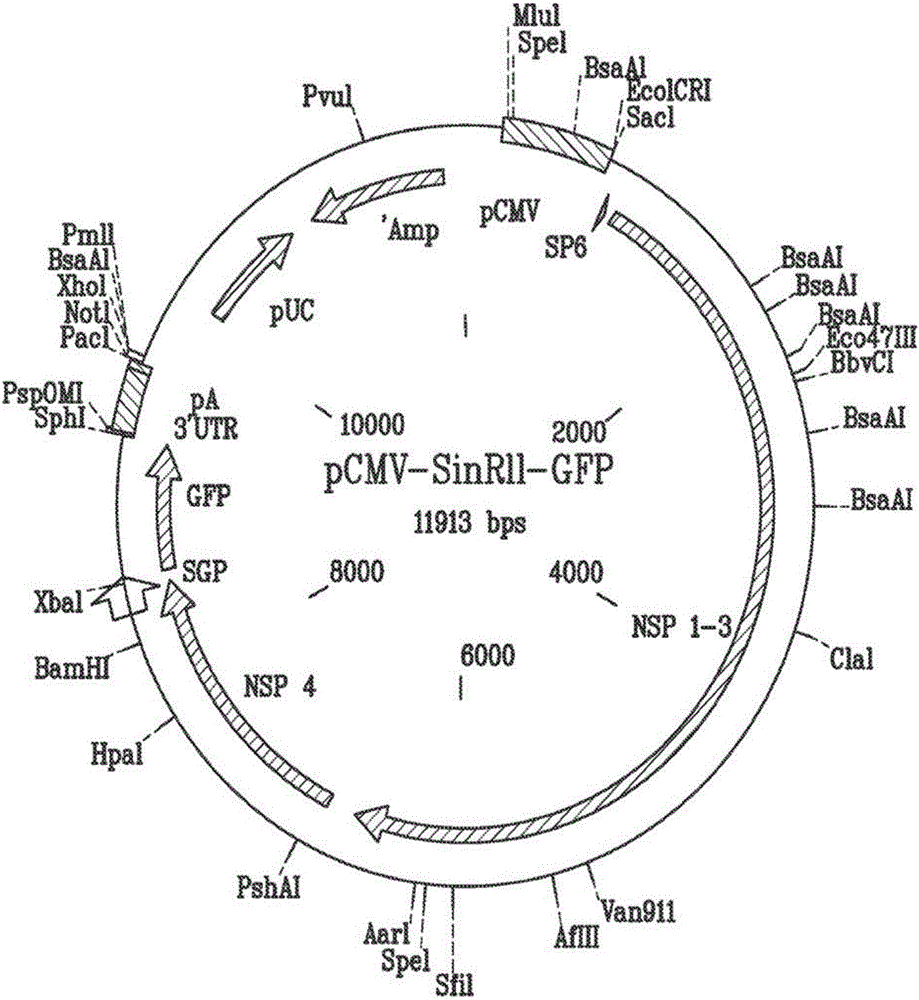
[0078] Example 1 - Generation of an alphavirus-based gene expression system
[0079] The alphavirus gene expression system is designed to allow VLP-mediated delivery of exogenous gene of interest (GOI) or protein of interest (POI) to target cells with low risk of causing cytopathic virus infection. An expression system was designed using three vectors that can be expressed in packaging cell lines to produce transduced VLPs. One vector encodes an alphavirus-based expression construct, another vector encodes a retroviral gag protein to facilitate VLP formation, and a third vector encodes a fusion protein to mediate fusion of VLPs to host cells. Additionally, the system is constructed to work without the presence of alphavirus structural proteins.
[0080] To achieve this, an alphavirus-based DNA plasmid was generated with the following: cytomegalovirus promoter (CMV); followed by a retroviral packaging signal for the corresponding retroviral packaging protein GAG; followed by a...
Embodiment 2
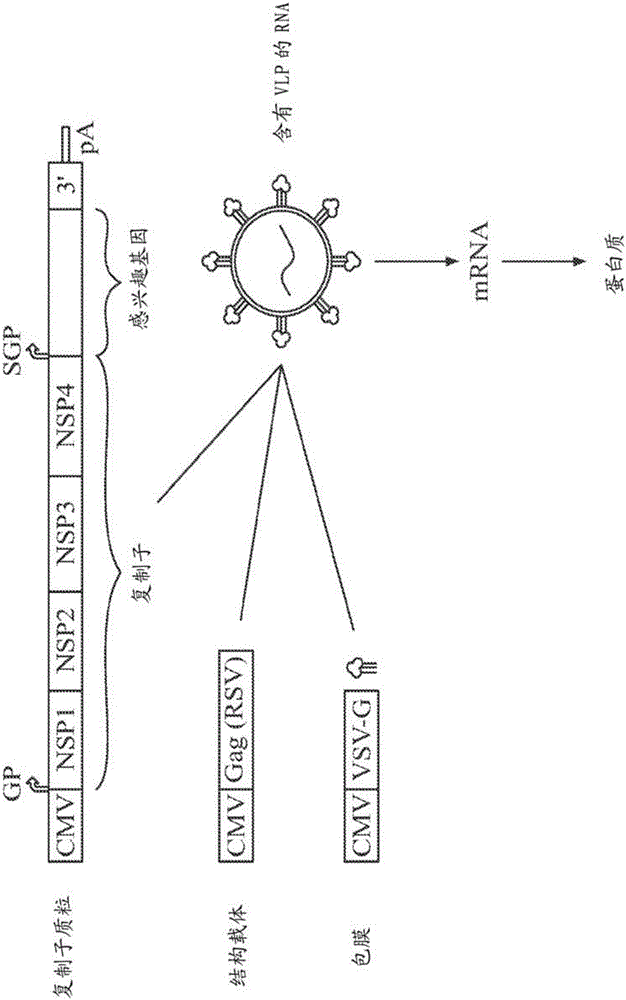
[0086] Example 2 - Expression of various proteins in target cells transduced with VEE VLPs
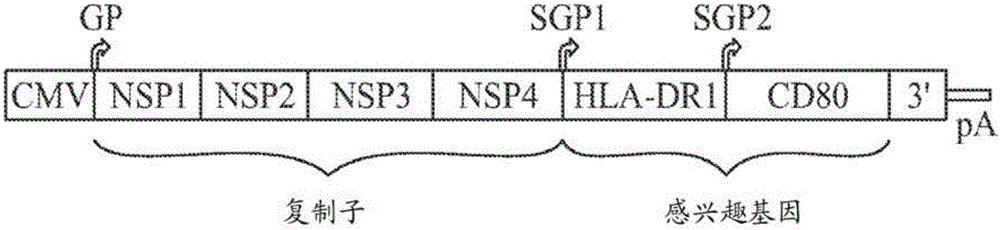
[0087] The basic concept of RNA delivery using VLPs is shown in figure 2 . In this experiment, a breast cancer model was used. A dual-promoter vector of the replicon was developed for the expression of HLA-DR1 and CD80 ( image 3 ). 4T1-Luc2 breast cancer cells were infected with VLPs encoding these two antigens. Using anti-HLA-DR and CD80 antibodies, it was shown that both proteins are indeed expressed in this cell. HLA-DR1 and CD80 have figure 2 Expression of the VLP-VEE replicon in VLP-transfected 4T1-Luc2 cells. Cells expressing HLA-DR1 were identified by using FITC-labeled anti-HLA-DR antibody. Cells expressing CD80 were identified by using PE-labeled anti-CD80 antibody.
[0088] To assess whether alphavirus replicons are capable of expressing two separate proteins in the same cell, VEE replication with HLA-DR1 under the control of one subgenomic promoter and CD80 under ...
Embodiment 3
[0089] Example 3 - Expression of various proteins in target cells transduced with VEE VLPs
[0090]To test VLPs in the mouse animal system, 4T1-Luc2 cells (highly aggressive and metastatic breast cancer cells) were implanted into the mammary fat pad of 10 mice. Tumors were visible within a few days and became apparent and palpable (5-7mm) within a week. Three mice were injected intratumorally with VLPs expressing inert protein (GFP), two mice were injected with VLPs expressing human HLA-DR1 / CD80 (allogeneic), and five mice remained uninjected as controls.
[0091] After 1 week, tumors were surgically removed from all mice and tumor regrowth and metastasis were followed over several weeks. result( Figure 5 ) showed that 4 out of 5 uninjected mice (untreated controls) died within 4-6 weeks due to cancer regrowth and metastasis in the lung, mammary gland and liver. All 3 mice injected with VLP / GFP (non-specific protein control) also died within 4-6 weeks due to cancer regrowt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com