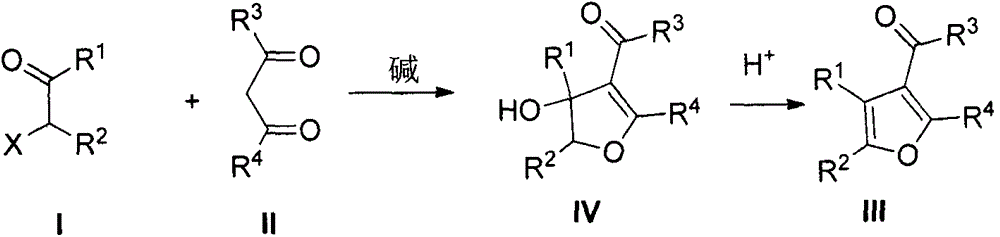
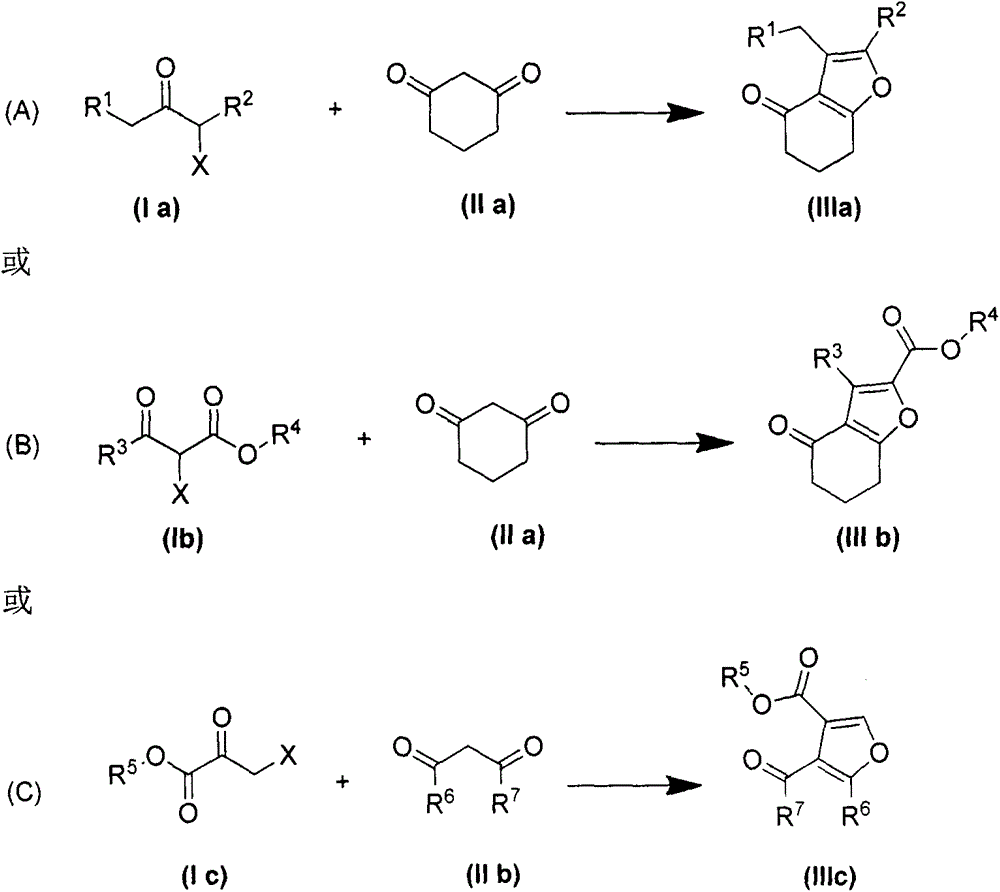
Method for preparing poly-substituted furan through Feist-Benary reaction under alkali-free and solvent-free condition
A multi-substituted furan, solvent-free technology, applied in the field of efficient synthesis of multi-substituted furan compounds, can solve the problems of complicated operation, low yield and the like, and achieves the effects of good regioselectivity, reduced production cost, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
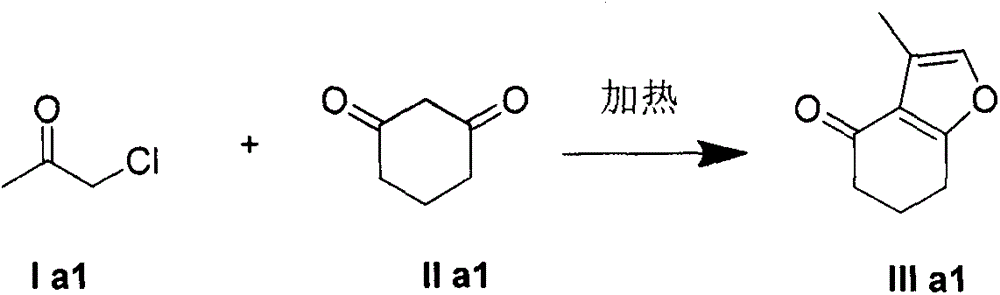
Embodiment 1
[0023] Example 1: 1-chloroacetone reacts with 1,3-cyclohexanedione
[0024]
[0025] In a 50 mL round bottom flask, 1-chlorocyclohexanone (10 mmol) and 1,3-cyclohexanedione (5 mmol) were added. Slowly heated up to 60°C while stirring, the mixture became a homogeneous liquid, and the reaction was continuously monitored by TLC during the reaction. After the reaction, the mixture obtained was directly separated by silica gel column chromatography to obtain furan IIIa1 as a yellow oil with a yield of 85%.
[0026] 1 H NMR (400MHz, CDCl 3 )δ7.06(s, 1H), 2.82(t, J=6.3Hz, 2H), 2.46(dd, J=7.2, 5.8Hz, 2H), 2.19(d, J=1.3Hz, 3H), 2.18- 2.10(m, 2H); 13 C NMR (101MHz, CDCl 3 )δ195.73, 167.42, 138.93, 120.44, 119.10, 38.31, 23.64, 22.76, 9.09; IR (KBr, cm -1 ) 2952, 1667, 1462, 1411, 1071, 573; MS (ESI) calcd for C 9 h 11 o 2 (M+H) + : 151.1, Found: 151.2.
Embodiment 2
[0027] Example 2: Reaction of ethyl 3-bromopyruvate with 1,3-cyclohexanedione
[0028]
[0029] In a 50 mL round bottom flask, ethyl 3-bromopyruvate (10 mmol) and 1,3-cyclohexanedione (5 mmol) were added. While stirring, the mixture was slowly heated up to 80°C and the mixture became a homogeneous liquid, and the reaction was continuously monitored by TLC during the reaction. After the reaction, the mixture obtained was directly separated by silica gel column chromatography to obtain furan IIIc1 as light yellow oil with a yield of 76%.
[0030] 1 H NMR (400MHz, CDCl 3 )δ7.85(s, 1H), 4.31(q, J=7.1Hz, 2H), 2.88(t, J=6.3Hz, 2H), 2.61-2.44(m, 2H), 2.24-2.07(m, 2H ), 1.34(t, J=7.1Hz, 3H); 13 C NMR (101MHz, CDCl 3)δ191.98, 168.33, 161.91, 147.77, 118.73, 117.48, 60.94, 38.71, 23.60, 22.17, 14.19; IR (KBr, cm -1 ): 3451, 3120, 1736, 1554, 1455, 1300, 1183, 1002, 878, 769, 586; MS (ESI) calcdfor C 11 h 13 o 4 (M+H) + : 209.0, Found: 209.1
Embodiment 3
[0031] Example 3: Reaction of ethyl 3-bromopyruvate with 1,3-pentanedione
[0032]
[0033] In a 50 mL round bottom flask, ethyl 3-bromopyruvate (10 mmol) and 1,3-pentanedione (5 mmol) were added. While stirring, the mixture was slowly heated up to 100°C and the mixture became a homogeneous liquid, and the reaction was continuously monitored by TLC during the reaction. After the reaction, the mixture obtained was directly separated by silica gel column chromatography to obtain furan IIIc2 as a yellow oil with a yield of 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com