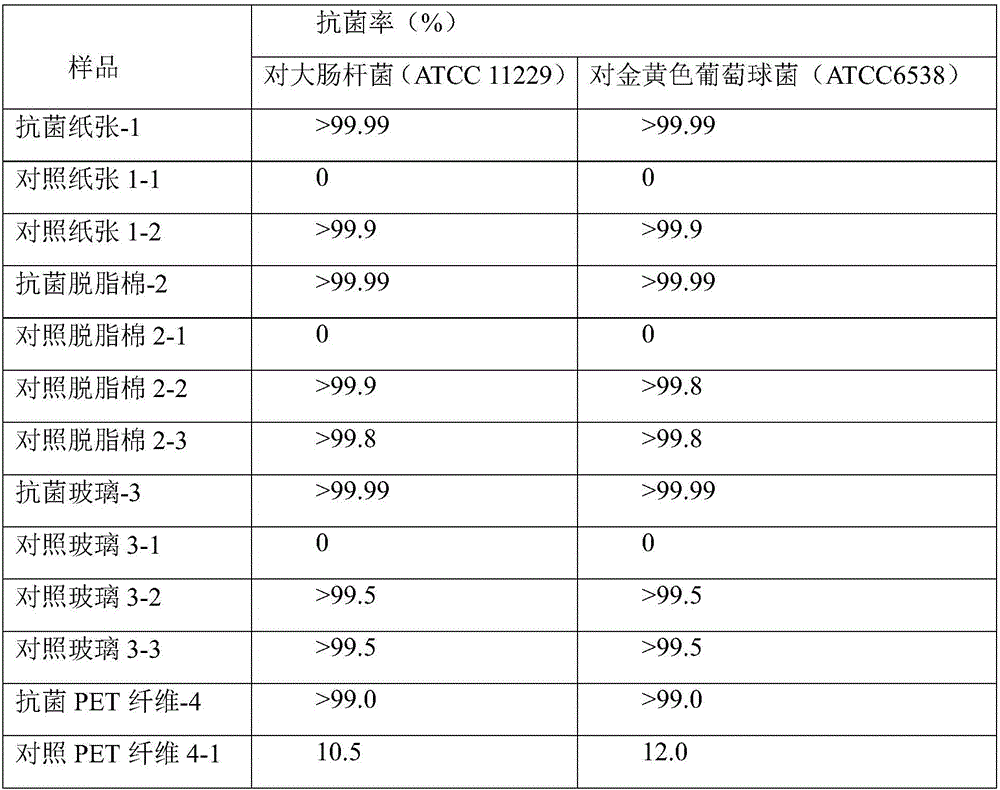
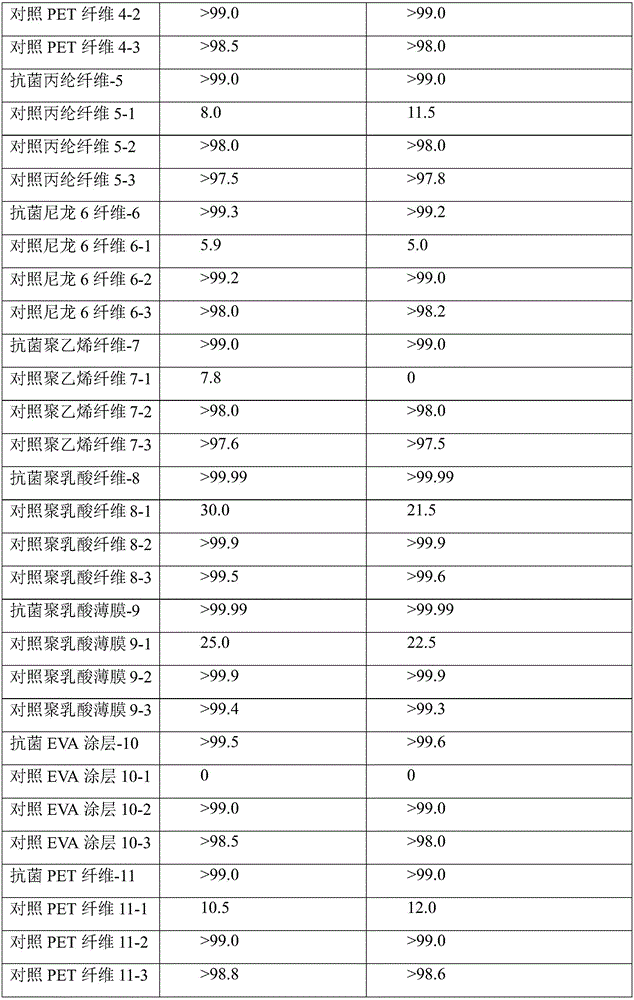
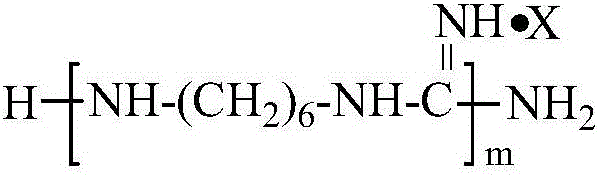Preparation method and applications of antibacterial guanidine salt copolymer
An antibacterial guanidine and copolymer technology, applied in the field of polymer synthesis, can solve complex problems and achieve the effect of convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 21 grams (0.03 moles) of polyhexylguanidine hydrochloride (A1, number average molecular weight 700Da) into a Haake rheometer at a temperature of 120°C, and after melting, add 38.4 grams of polypropylene glycol diglycidyl ether (B1, number Average molecular weight 640Da, 0.06 mole), under the condition of rotating speed 60 rpm, reacted for 60 minutes, obtained the block copolymer of polyhexylguanidine hydrochloride-polypropylene glycol diglycidyl ether, marked as B1A1B1.
Embodiment 2
[0022] Add 23.4 grams (0.0275 moles) of polyhexylguanidine phosphate (A2, number average molecular weight 850Da) into a Haake rheometer at a temperature of 130°C, and after melting, add 35.2 grams of polypropylene glycol diglycidyl ether (B1, number average Molecular weight 640Da, 0.055 mol), under the condition of rotating speed 60 revs / min, react for 50 minutes, obtain the block copolymer of polyhexylguanidine phosphate-polypropylene glycol diglycidyl ether, marked as B1A2B1.
Embodiment 3
[0024] 14 grams (0.02 moles) of polyhexylguanidine hydrochloride (A1, number average molecular weight 700Da) was dissolved with 100 grams of dimethyl sulfoxide to form a solution and then added to a 250ml three-necked flask, and polypropylene glycol diglycidyl ether 19.2 Gram (B1, number average molecular weight 640Da, 0.03 moles), heated to 40°C for 120 minutes under magnetic stirring to obtain a polyhexylguanidine hydrochloride-polypropylene glycol diglycidyl ether block copolymer solution. Then, the solvent was removed by dialysis in water for 48 hours using a dialysis bag with a molecular weight cut off of 1000, to obtain an aqueous dispersion of the block copolymer. The resulting block copolymer is labeled B1A1B1A1B1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com