4-terpineol fatty ester derivate as well as application and preparation method thereof
A technology of fatty acid ester and terpineol, which is applied in the fields of 4-terpineol fatty acid ester derivatives and their application and preparation, can solve the problems of poor reproducibility, unstable content and the like, and achieves easy preservation, enhanced permeability and the like. Ability, the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
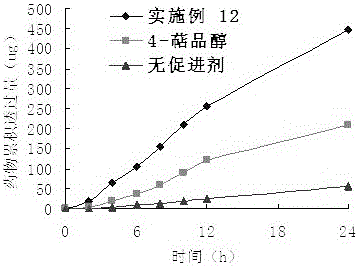
Embodiment 1
[0038] Take 14.8g (0.2mol) of propionic acid, add 17.85g (0.15mol) of thionyl chloride, mix well and react at 60°C for 3 hours, add 20ml of 4-terpineol tetrahydrofuran solution containing 15.4g (0.1mol), The reaction was continued for 3 hours, the reaction solution was adjusted to pH 6-7 with NaOH solution, the organic layer was separated, the aqueous layer was extracted with ethyl acetate, the organic layers were combined, washed with saturated brine, and anhydrous Na 2 SO 4 Dry overnight, evaporate the solvent, perform column chromatography (silica gel is 200-300 mesh), use ethyl petroleum ether and ethyl acetate (15:1 volume ratio) as the eluent, evaporate the eluent, and obtain 18.64 g of a colorless liquid , Yield: 94%. product of 1 HNMR and MS data are as follows: ESI-MS m / z: 211.3 [M+1]+; 1 H NMR(CDCl3),: 0.90(3H, s), 1.01(3H, s), 1.0(3H, s), 1.25(3H, s), 1.30~1.35(2H, m), 1.40(1H, m) , 1.58~1.60(2H, m), 1.87(1H, m), 2.04(2H, m), 4.78(1H, d, J=11.2 Hz).
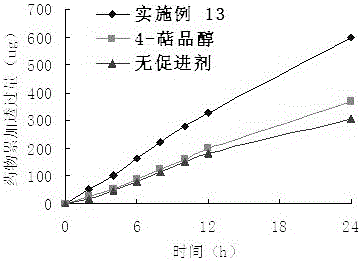
Embodiment 2
[0040] Take 17.6g (0.2mol) of butyric acid, add 17.85g (0.15mol) of thionyl chloride, mix and react at 60°C for 3 hours, add 20ml of tetrahydrofuran solution containing 15.4g (0.1mol) of 4-terpineol, The reaction was continued for 3 hours, the reaction solution was adjusted to pH 6-7 with NaOH solution, the organic layer was separated, the aqueous layer was extracted with ethyl acetate, the organic layers were combined, washed with saturated brine, and anhydrous Na 2 SO 4 Dry overnight, evaporate the solvent, perform column chromatography (silica gel is 200-300 mesh), use petroleum ether and ethyl acetate (15:1 volume ratio) as the eluent, evaporate the eluent, and obtain 20.91 g of a colorless liquid , rate: 92.4%. product of 1 HNMR and MS data are as follows: ESI-MS m / z: 225.3 [M+1] + ; 1 H NMR(CDCl3): 0.90(3H, s), 1.01(3H, s), 0.99(3H, s), 1.25(3H, s), 1.30~1.35(4H, m), 1.41(1H, m), 1.58~1.60(2H, m), 1.88(1H, m), 2.03(2H, m), 4.78(1H, d, J=11.2 Hz).
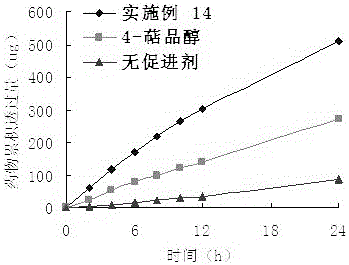
Embodiment 3
[0042] Take 23.2g (0.2mol) of hexanoic acid, add 17.85g (0.15mol) of thionyl chloride, mix well and react at 60°C for 3 hours, add 20ml of tetrahydrofuran solution containing 15.4g (0.1mol) of 4-terpineol, The reaction was continued for 3 hours, the reaction solution was adjusted to pH 6-7 with NaOH solution, the organic layer was separated, the aqueous layer was extracted with ethyl acetate, the organic layers were combined, washed with saturated brine, and anhydrous Na 2 SO 4 Dry overnight, evaporate the solvent, perform column chromatography (silica gel is 200-300 mesh), use ethyl petroleum ether and ethyl acetate (15:1 volume ratio) as the eluent, evaporate the eluent, and obtain 23.94 g of a colorless liquid , Yield: 93.4%. product of 1 HNMR and MS data are as follows: ESI-MS m / z: 253.4 [M+1] + ; 1 H NMR(CDCl3): 0.90(3H, s), 1.00(3H, s), 1.01(3H, s), 1.24 (3H, s), 1.30~1.35(8H, m), 1.40(1H, m), 1.58~1.60(2H, m), 1.89(1H, m), 2.04 (2H, m), 4.78(1H, d, J=11.2 Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com