Water-soluble florfenicol clathrate with high bioavailability and preparation method of water-soluble florfenicol clathrate
A florfenicol and availability technology, which is applied in the directions of drug combinations, medical preparations of inactive ingredients, pharmaceutical formulations, etc., can solve the problem of low oral bioavailability, poor water solubility of florfenicol, and low bioavailability. problems, to achieve the effect of masking bad odor, improving solubility, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
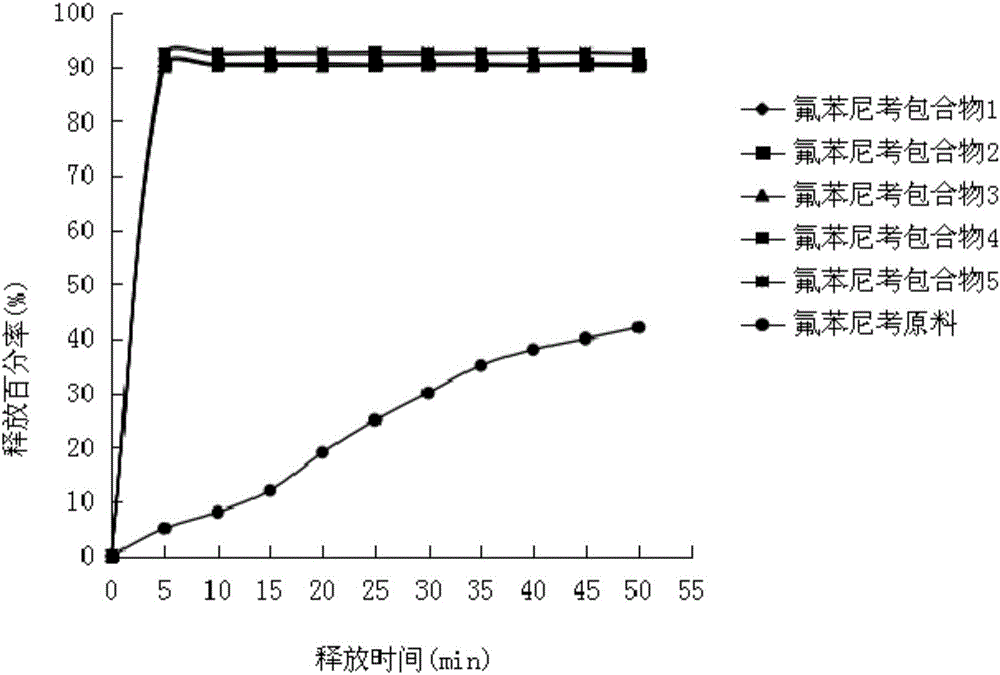
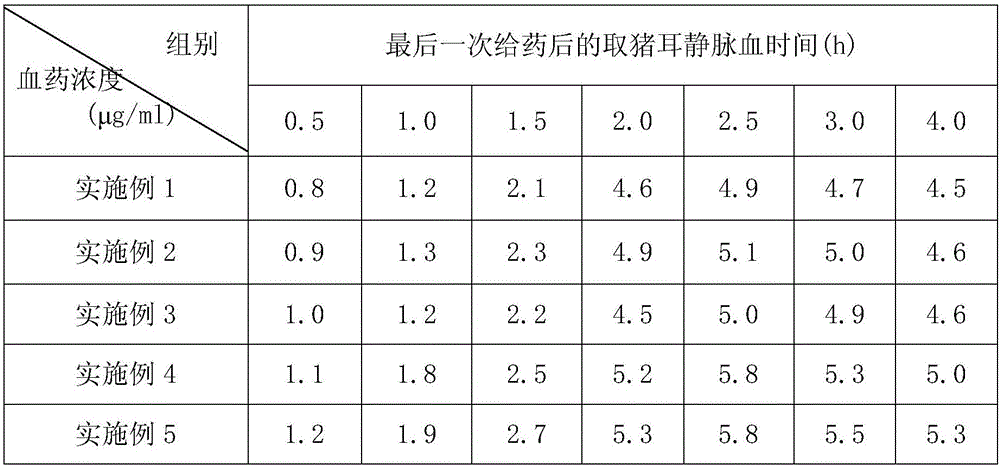
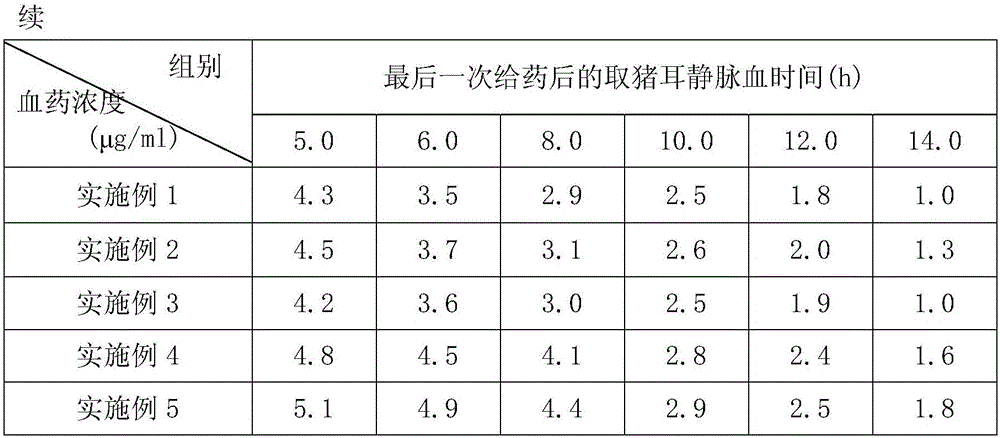
Image
Examples
Embodiment 1
[0045] Preparation of florfenicol inclusion compound
[0046]Weigh 2.0kg of hydroxypropyl-sulfobutyl-β-cyclodextrin, 0.05kg of methylcellulose, 0.05kg of hydroxypropylcellulose, 0.05kg of poloxamer, 0.05kg of polyvinylpyrrolidone, and add to 60°C Continue stirring in 5L of water to dissolve it to obtain mixture 1; place mixture 1 in a water bath at 60°C, slowly add 0.5kg of florfenicol, and stir rapidly for 30 minutes until a uniform colorless solution is formed to obtain mixture 2; continue Stir under temperature control and keep warm for 3 hours. After the keep warm is over, prepare the mixture 2 into a powder by freeze-drying to obtain florfenicol inclusion compound 1. The freeze-drying conditions are: put the mixture 3 in batches into the refrigerator to freeze , the freezing time is 24 hours, then placed in a vacuum freeze dryer, vacuumed to about 1.3pa, and continued to dry for 18 hours.
Embodiment 2
[0048] Preparation of florfenicol inclusion compound
[0049] Weigh 1.0kg of hydroxypropyl-sulfobutyl-β-cyclodextrin, 1.0kg of ethylenediamine-β-cyclodextrin, 0.05kg of Tween-800.05kg, 0.05kg of disodium edetate, salicyl Add 0.05kg of sodium p-aminobenzoate and 0.05kg of sodium p-aminobenzoate into 5L of water at 60°C and continue stirring to dissolve to obtain mixture 1; place mixture 1 in a water bath at 60°C, and slowly add 0.5kg of florfenicol sodium succinate , stirred rapidly for 30 minutes, until a uniform colorless solution was formed, and mixture 2 was obtained; continue to control the temperature and stir, keep warm for 3 hours, and when the heat preservation is over, prepare the mixture 2 into powder by spray drying, and obtain the inclusion compound of florfenicol 2. The spray drying conditions are: the inlet air temperature is 160°C, the outlet air temperature is 40°C, the spray pressure is 0.6Mpa, and the vacuum degree is 0-10Kpa.
Embodiment 3
[0051] Preparation of florfenicol inclusion compound
[0052] Weigh 1.0kg of ethylenediamine-β-cyclodextrin, 1.0kg of sulfobutyl-β-cyclodextrin, 0.05kg of methylcellulose, 0.05kg of sodium lauryl sulfate, 0.05kg of polyvinylpyrrolidone, Add 0.05kg of sodium salicylate to 5L of water at 60°C and continue to stir to dissolve it to obtain mixture 1; put mixture 1 in a water bath at 60°C, slowly add 0.5kg of florfenicol sodium phosphate, and stir rapidly for 30 minutes , to form a uniform colorless solution, to obtain mixture 2; continue to control temperature and stir, keep warm for 3 hours, and heat preservation is over, prepare mixture 2 into powder by spray drying, that is, obtain florfenicol inclusion compound 3, spray drying conditions It is: the inlet air temperature is 160°C, the outlet air temperature is 40°C, the spray pressure is 0.6Mpa, and the vacuum degree is 0-10Kpa.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com