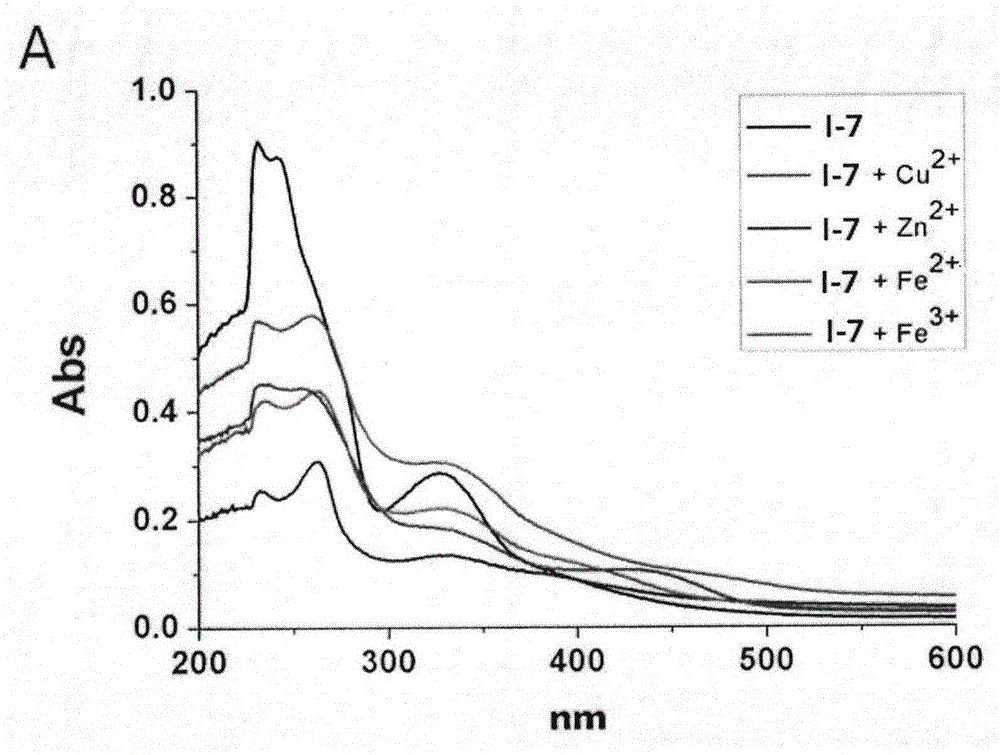
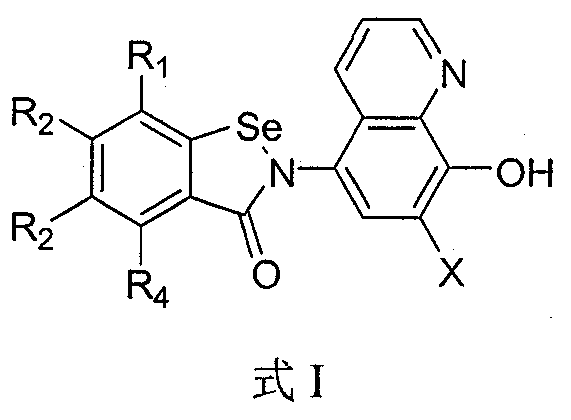
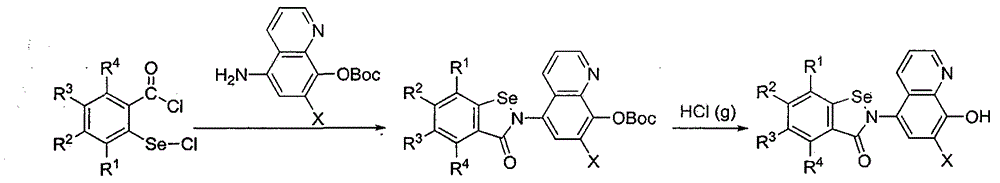
Benzisoselenazolone quinoline derivative and application thereof in treatment of Alzheimer's disease
A technology of compounds and hydrates, which is applied in the field of medicine, can solve the problems of no new drugs being successfully marketed and no breakthroughs, etc., to achieve the ability to inhibit metal ion chelation, inhibit Aβ aggregation, and excellently simulate glutathione process. Effect of oxidase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 5-nitro-7-chloro-8-hydroxyquinoline
[0024]
[0025] 5-Nitro-8-hydroxyquinoline (4.8g, 25.2mmol) and KOH solution (1mol / L, 25.2mL) were added to 480mL deionized water, and NaClO solution (7%, 34.2mL ). During the dropwise addition, the raw material was completely dissolved, and then a solid precipitated out. Acetic acid was added dropwise to adjust the solution to pH=6. Suction filtration and vacuum drying yielded 4.0 g of a yellow solid. Melting point, 239°C. Yield 71%
Embodiment 2
[0026] Example 2 5-nitro-7-iodo-8-hydroxyquinoline
[0027]
[0028] 5-Nitro-8-hydroxyquinoline (10.0g, 52.6mmol) and KOH (9g, 160.0mmol) were added to 1000mL deionized water, and evenly mixed I 2 (13.35g, 52.6mmol) and KI (8.73g, 52.6mmol) were solid. After stirring at room temperature for 2h, acetic acid was added dropwise to adjust the solution to pH=6. Suction filtration and vacuum drying yielded 10.8 g of a yellow solid. Melting point, 236°C. Yield 65%
Embodiment 3
[0029] Example 3 tert-butyl-(5-nitro-8-hydroxyquinoline) carbonate
[0030]
[0031] Dissolve 5-nitro-8-hydroxyquinoline (10.0g, 52.6mmol) and di-tert-butyl carbonate (12.7g, 57.8mmol) in n-hexane / dichloromethane (420mL, V / V=1:2) 4-N,N-dimethylaminopyridine (0.65 g, 5.26 mmol) and diisopropylethylamine (11.2 mL, 63.12 mmol) were added under stirring at room temperature. After stirring for 14h, it was filtered with suction and concentrated. Purified by column chromatography to obtain 14.0 g of light yellow solid. Yield 92%. 1 H NMR (400MHz, CDCl 3 )δ9.06(d, J=8.9Hz, 1H), 9.03(s, 1H), 8.44(d, J=8.4Hz, 1H), 7.68(dd, J=5.5, 3.3Hz, 1H), 7.62( d, J=8.4Hz, 1H), 1.60(s, 19H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com