Multifunctional acetylcholinesterase inhibitor and its application
A technology of acetylcholinesterase and inhibitors, applied in the field of new multifunctional acetylcholinesterase inhibitors, can solve the problems of poor therapeutic effect of AChE inhibitors, achieve obvious neuroprotective effects, and inhibit Aβ aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
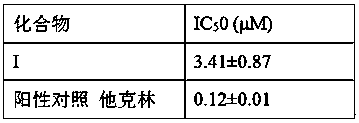
[0024] Example 1 Effect of compound on AChE enzyme activity
[0025] Acetylcholinesterase (AChE), acetylthiocholine iodide (ATC) as a substrate and 5,5-dithiobis(2-nitrobenzoic acid) (DTNB) as a developer were all purchased from Sigma. The AChE inhibitory activity was determined with reference to the method reported by Ellan et al. (Ellman, G. L. et al. Biochem. Pharmacol. 1961, 7, 88.). Add 40 μL of phosphate buffer (pH = 8.0) to each well of a 96-well plate, and then add 0.39, 0.78, 1.56, 3.125, 6.25, 12.5, 25, 50 and 100 μM of 10 μL of the test compound solution or blank control to the corresponding Then add 10 μL of AChE and incubate in a shaker at 37°C for 5 min. Add 20 μL of DTNB solution, then place it in a 37°C shaker and incubate for 5 min, then add 10 μL of substrate ATC, place it in a 37°C shaker and incubate for 3 min, and then measure the absorbance at 412 nm with a microplate reader and calculate Inhibition rate of the test compound to AChE. Calculate the IC of...
Embodiment 2
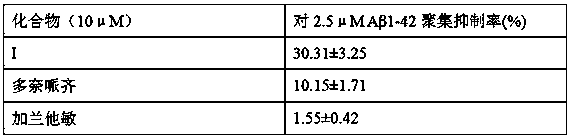
[0028] Example 2 Effect of compound on Aβ aggregation
[0029] Use DMSO (Sigma) to combine Aβ 1-42 (Gill Biochemical Co., Ltd., Shanghai, China) was dissolved and prepared into a 200 μM stock solution. The stock solution was centrifuged at 13,500 rpm for 10 minutes, and the supernatant of the stock solution was used for the experiment. Dissolve the test compound to 0.8 mM with DMSO. The ability of the compound to inhibit Aβ aggregation was indirectly tested by measuring the fluorescence emission of ThT. First add 76μL of phosphate buffered saline (pH 7.4 PBS) to the 96-well plate, then add 2μL of 0.8 mM compound and 2μL of 200μM Aβ 1-42 After incubating for 24 hours at room temperature, add 80 μL of 5 μM ThT solution (dissolve THT with 50 mM glycine-NaOH buffer pH 8.5) to the reaction solution. The fluorescence emission was measured at 490 nm of the microplate reader, and the excitation wavelength was 390 nm. The same spectrum was recorded by performing three independent exper...
Embodiment 3
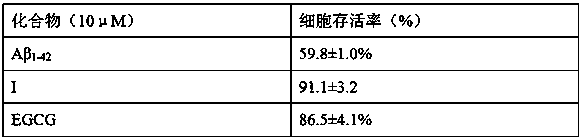
[0033] Example 3 Compound vs. Aβ 1-42 The protective effect of inducing SH-SY5Y nerve cell damage
[0034] SH-SY5Y was purchased from the National Cell Bank of the United States, using DMEM medium containing 10% fetal bovine serum, and routinely cultured in an incubator at 37°C and 5% CO2 saturated humidity. Take 2-2.5×10 5 The density of cells / mL was seeded in 96-well plates, and the experiment was carried out after 24 hours of culture. Replace with fresh culture medium and divide the cells into normal group, Aβ 1-42 Damage model group and test compound pretreatment group. Compounds of formula I (10 µM) were added to the test compound treatment group, EGCG (tea polyphenol) was added to the EGCG positive control group, and the Aβ group and the normal group were not added. After culturing for 2 hours, add Aβ at a final concentration of 10 µM to all groups except the normal group 1-42 , Continue to incubate for 24 hours, add MTT (final concentration 0.5 mg / ml), after incubating fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com