6-bromotryptamine derivative as well as preparation method and application thereof
A technology of bromotryptamine and its derivatives, which is applied in the field of 6-bromotryptamine derivatives and its preparation, and can solve the problems of low activity and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A 6-bromotryptamine derivative, the structural formula is represented by (I):
[0028] ..... (Ⅰ)
[0029] In the general formula (I), R is selected from -Cl or -H;
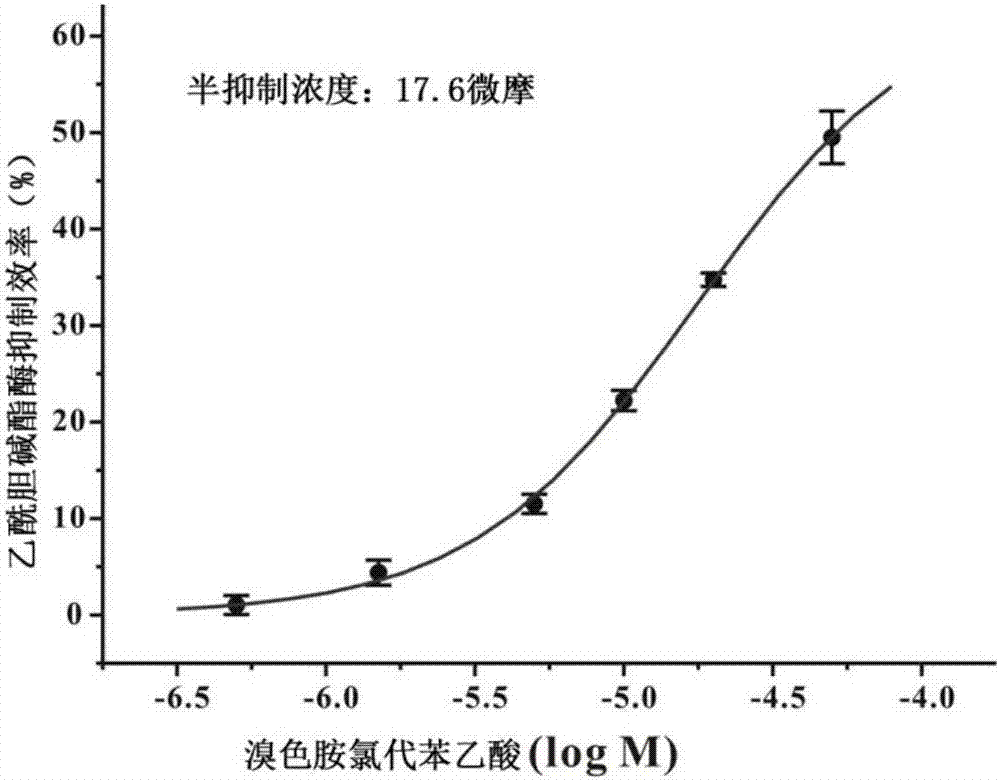
[0030] When R=-Cl, the compound is 6-bromotryptamine chlorophenylacetic acid;
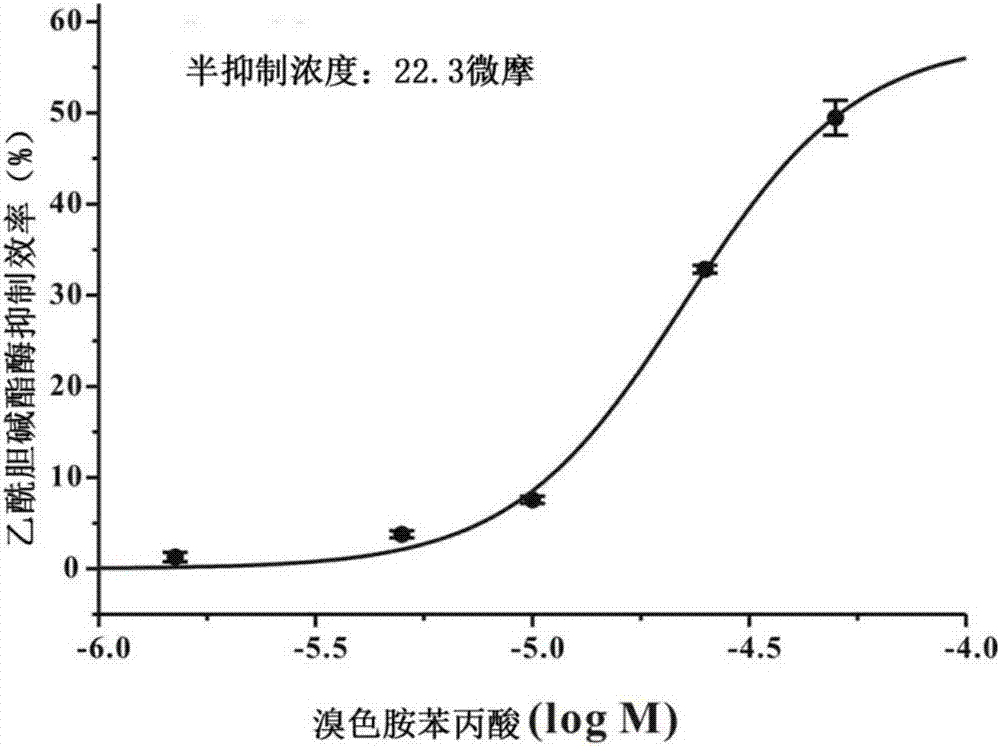
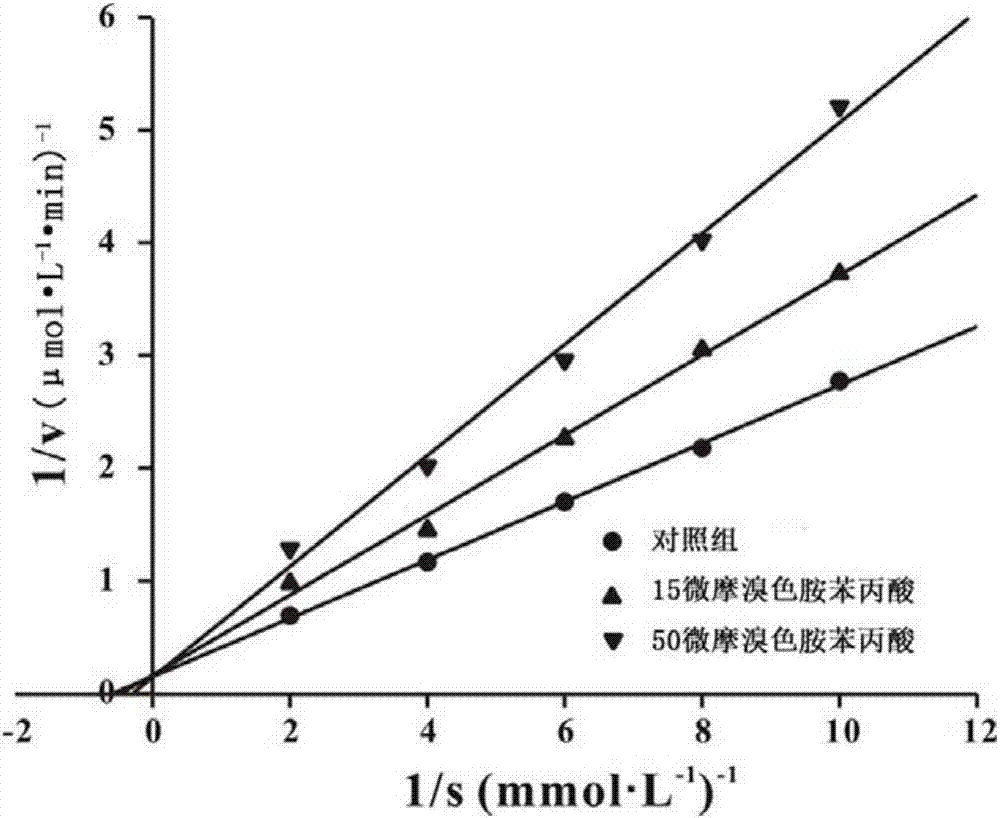
[0031] When R=-H, the compound is 6-bromotryptamine phenylpropionic acid.
Embodiment 2
[0033] Preparation of Bromotryptamine Chlorophenylacetic Acid
[0034] Weigh 0.35mmol of chlorophenylacetic acid, dissolve it in 5mL of dichloromethane, then add 0.096g of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC.HCL), and White turbid liquid, then add 0.068g 1-hydroxybenzotriazole (HOBT), stir at room temperature for 30min, the white turbidity disappears, check the reaction process with thin-layer chromatography, and when new spots appear, dissolve 7mL with 0.25 Mmol 6-bromotryptamine (where 6-bromotryptamine is 6-bromotryptamine hydrochloride obtained by removing hydrochloric acid with sodium hydroxide) was added dropwise to the above reaction solution in dichloromethane, stirred at room temperature overnight, and was analyzed by TLC After detection, it was found that the raw material point disappeared, the reaction was stopped, the solvent was distilled off, and the crude product was obtained, and a small amount of product was finally separated by semi...
Embodiment 3
[0037] Preparation of 6-bromotryptamine phenylpropionic acid
[0038] Weigh 0.35mmol of phenylpropionic acid, dissolve it in 5mL of dichloromethane, then add 0.096g of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC.HCL), a white To the turbid liquid, add 0.068g 1-hydroxybenzotriazole (HOBT), stir at room temperature for 30min, the white turbidity disappears, detect the reaction process with thin-layer chromatography, and when new spots appear, dissolve 7mL with 0.25mmol 6-Bromotryptamine dichloromethane was added dropwise to the above reaction solution, stirred at room temperature overnight, detected by TLC, found that the raw material point disappeared, stopped the reaction, evaporated the solvent to obtain the crude product, and finally used semi-preparative liquid chromatography Trace product was isolated. Semi-preparative liquid chromatography conditions: dissolve the crude product in methanol, filter with a pore size of 0.22 μm, use an XBridge preparative...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com