A kind of acetylcholinesterase inhibitor and application thereof
A technology of acetylcholinesterase and inhibitors, applied in the field of acetylcholinesterase inhibitors, can solve the problems of poor therapeutic effect of AChE inhibitors, achieve obvious neuroprotective effects, and inhibit Aβ aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
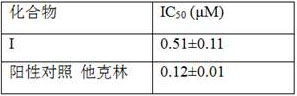
[0024] Embodiment 1 The influence of compound on AChE enzymatic activity
[0025] Acetylcholinesterase (AChE), thioacetylcholine iodide (ATC) as a substrate, and 5,5-dithiobis(2-nitrobenzoic acid) (DTNB) as a chromogenic reagent were purchased from Sigma. The determination of AChE inhibitory activity was carried out with reference to the method reported by Ellan et al. (Ellman, G. L. et al. Biochem. Pharmacol. 1961, 7, 88.). Add 40 μL of phosphate buffer (pH = 8.0) to each well of the 96-well plate, and then add 0.39, 0.78, 1.56, 3.125, 6.25, 12.5, 25, 50 and 100 μM of 10 μL of the test compound solution or blank control to the corresponding Then add 10 μL of AChE and incubate at 37°C on a shaker for 5 min. Add 20 μL of DTNB solution and incubate at 37°C for 5 min, then add 10 μL of substrate ATC and incubate at 37°C for 3 min, measure the absorbance at 412 nm with a microplate reader, and calculate Inhibition rate of test compound on AChE. Calculate the IC of the compoun...
Embodiment 2
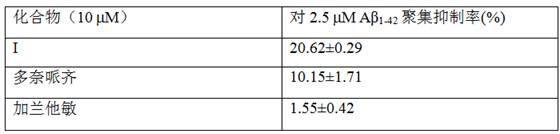
[0028] Example 2 Effects of Compounds on Aβ Aggregation
[0029] Aβ was treated with DMSO (Sigma) 1-42 (Gill Biochemical Co., Ltd., Shanghai, China) was dissolved to prepare a 200 μM stock solution. The stock solution was centrifuged at a speed of 13,500 rpm for 10 minutes, and the supernatant of the above stock solution was taken for the experiment. Test compounds were dissolved in DMSO to 0.8 mM. The ability of compounds to inhibit Aβ aggregation was tested indirectly by measuring the fluorescence emission of ThT. Add 76 μL of phosphate-buffered saline (PBS, pH 7.4) to a 96-well plate, then add 2 μL of 0.8 mM compound and 2 μL of 200 μM Aβ 1-42 , after incubation at room temperature for 24 h, 80 μL of 5 μM ThT solution (THT was dissolved with 50 mM glycine-NaOH buffer at pH 8.5) was added to the reaction solution. Fluorescence emission was measured at 490 nm on a microplate reader with an excitation wavelength of 390 nm. The same spectra were recorded by performing thre...
Embodiment 3
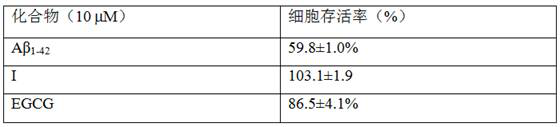
[0033] Example 3 Compounds on Aβ 1-42 Induced protective effect of SH-SY5Y nerve cell injury
[0034] SH-SY5Y was purchased from the National Cell Bank of the United States, and was routinely cultured in DMEM medium containing 10% fetal bovine serum in an incubator at 37°C and 5% CO2 saturated humidity. Take 2-2.5×10 5 Cells / mL were seeded in 96-well plates, and experiments were performed after 24 h of culture. Replace with fresh culture medium, and divide the cells into normal group, Aβ 1-42 Injury model group, test compound pretreatment group. Compounds of formulas I, II and III (10 μM) were added to the test compound treatment group, EGCG (tea polyphenols) was added to the EGCG positive control group, and no addition was made to the Aβ group and the normal group. After culturing for 2 h, Aβ at a final concentration of 10 μM was added to each group except the normal group 1-42 , continue to culture for 24h, add MTT (final concentration 0.5 mg / ml), after culture for 3 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com