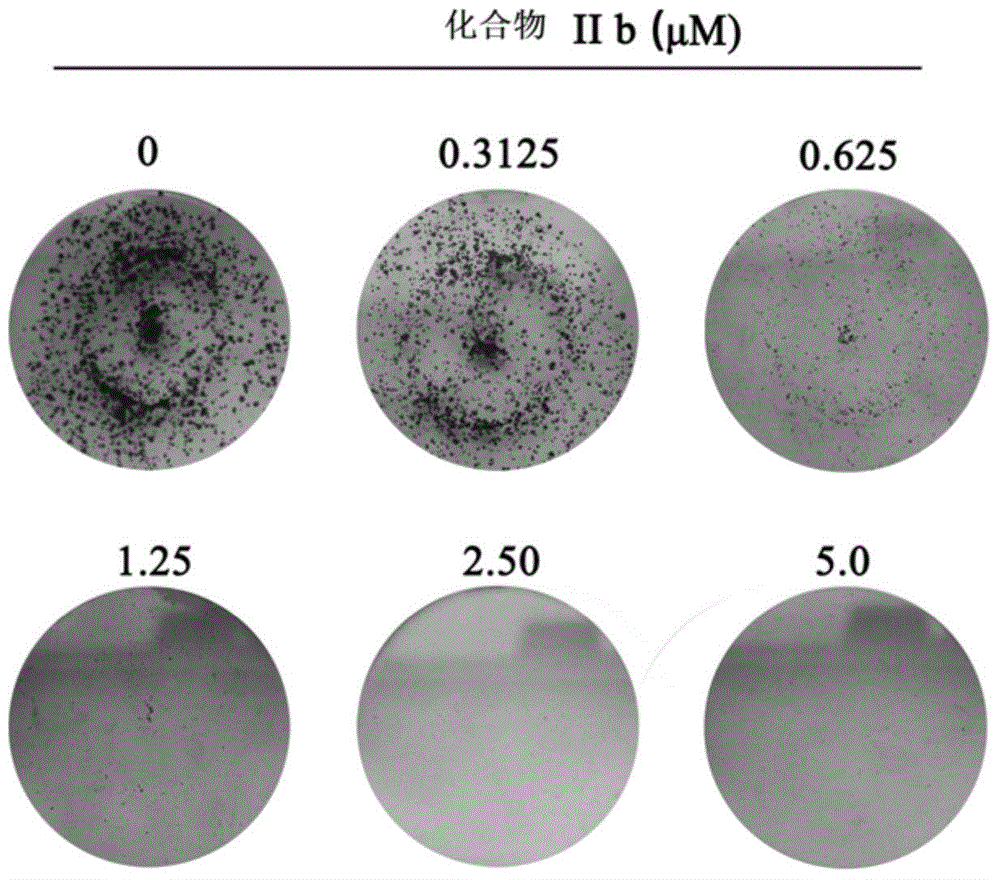
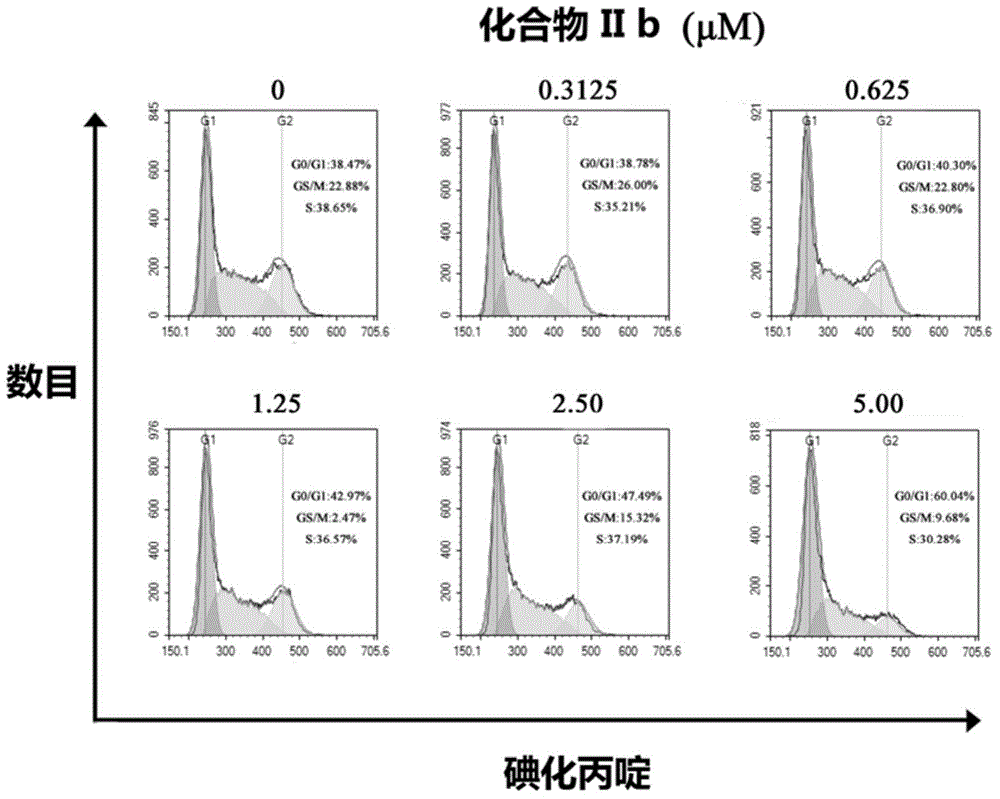
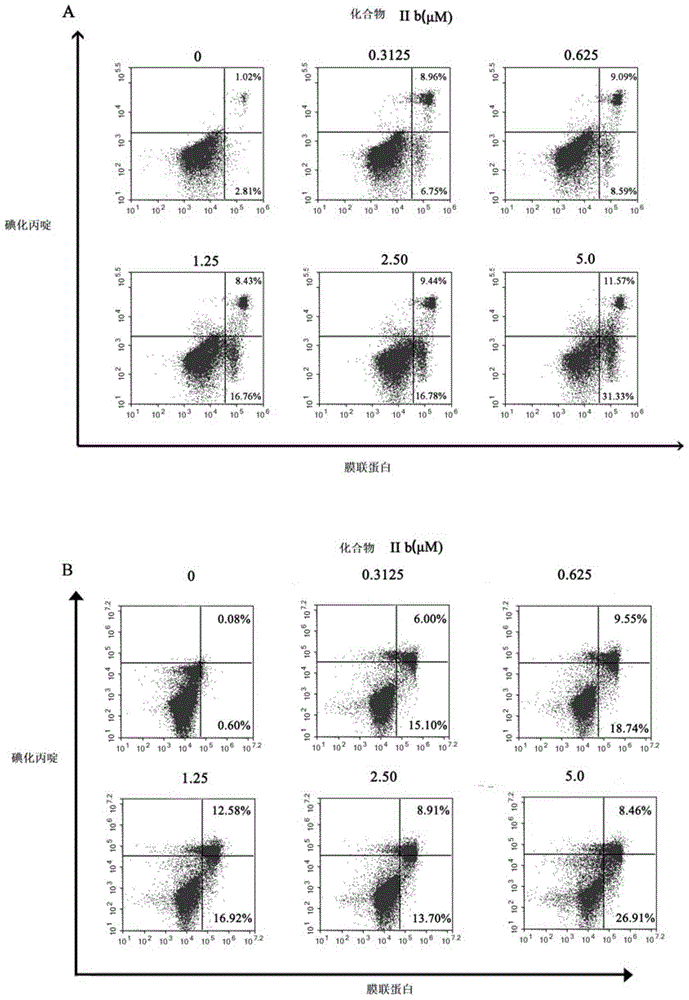
Coumarinoimidazole derivative or pharmaceutically acceptable salt thereof, and uses of coumarinoimidazole derivative or pharmaceutically acceptable salt thereof
A technology of medicinal salts and halogens, applied in the field of chemical medicine, can solve the problems of low selectivity, damage to normal cells, large toxic side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Compound Ia: the structural formula is Its preparation method is:
[0036] First use the method disclosed in the application number 201310205309.X to prepare the compound
[0037] The preparation method of compound Ia:
[0038] Dissolve 5-phenyl-4-oxo-7-bromo-3,4-dihydroimidazo[4,5-c]coumarin (375 mg, 1 mmol) and potassium tert-butoxide (336 mg, 3 mmol) in In 2mL DMF, slowly add CH dissolved at 0°C dropwise 3 The DMF solution of 2mL of I (142mg, 1mmol), TLC monitors; About 40 minutes, product generation is arranged, but there is reactant, if prolonging reaction time, product changes into by-product, and yield declines; Processing reaction: promptly reacted for 40 minutes After that, the reaction solution was introduced into water for dispersion, extracted three times with ethyl acetate, backwashed three times with saturated saline, dried over anhydrous sodium sulfate, spin-dried, and passed through the column with petroleum ether / ethyl acetate system to obtain 30 ...
Embodiment 2
[0042] Preparation of compound II a: the reaction principle is the same as in Example 1, and the compound is first prepared by the method disclosed in the application number 201310205309.X Referring again to the preparation method of Example 1, compound II a was obtained:
[0043]
[0044] 1 H NMR (400MHz, DMSO) δ7.55(d, J=8.3Hz, 2H), 7.44(dd, J=8.8, 2.3Hz, 1H), 7.37(d, J=2.3Hz, 1H), 7.29(d ,J=8.2Hz,2H),6.84(d,J=8.9Hz,1H),3.49(s,3H),2.18(s,3H),1.66(s,6H),1.23(s,9H)ppm. ESI-MS: 510,512[M+H] + .
Embodiment 3
[0046] Preparation of Compound Ib: Refer to Example 1 for the reaction raw materials.
[0047]
[0048] 1 H NMR (400MHz, DMSO) δ9.14(s, 1H), 8.52(s, 1H), 8.01(t, J=7.8Hz, 2H), 7.83(d, J=10.6Hz, 1H), 7.75(m ,2H),7.63(t,J=7.4Hz,1H),7.40(m,3H),7.30(d,J=7.4Hz,2H),7.05(d,J=8.6Hz,1H),3.63(s ,3H),2.21(s,3H),1.19(s,9H)ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com