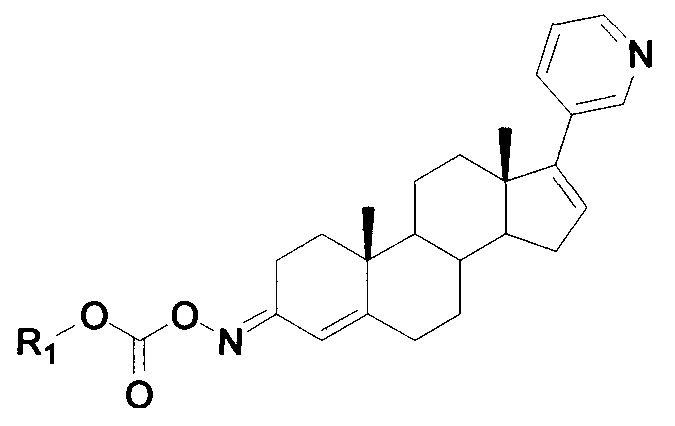
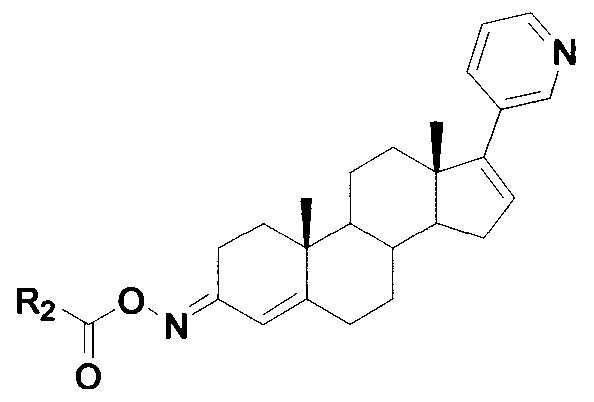
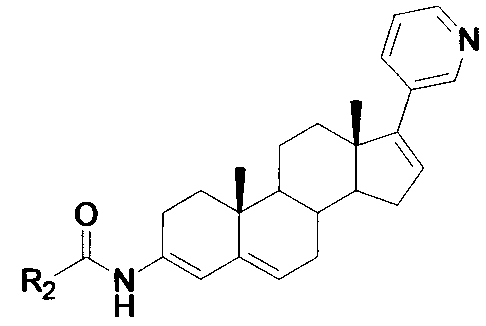
Compound serving as inhibitor for CYP11B, CYP17 and CYP21
A compound and composition technology, applied in the field of compounds as inhibitors of CYP11B, CYP17, and CYP21, can solve problems such as potency blocking, CYP11B1 activity potency limitation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] Example 1: Put 3.62g of SM in diethyl ether, add 2.2g of triethylamine, 1.2g of ethoxyformyl chloride, and react at room temperature for 2h. Add 50ml of EA, add 10ml of water to wash twice, dry the organic phase with anhydrous sodium sulfate, evaporate the organic solvent under reduced pressure, and obtain about 3.5g of the product crystallized from dichloromethane.
[0029]
example 2
[0030] Example 2: Put 3.62g SM in tetrahydrofuran, add 5.0g iron powder, add dropwise 0.95g acetyl chloride, and react at room temperature for 5h. Add 50ml of EA, add 10ml of water to wash twice, dry the organic phase with anhydrous sodium sulfate, evaporate the organic solvent under reduced pressure, and obtain about 2.7g of product crystallized from dichloromethane.
[0031]
example 3
[0032] Example 3: Add 0.95 g of acetyl chloride to 3.62 g of SM in ether, react at room temperature for 2 h, and filter. 50ml EA was added to the mother liquor, 10ml water was added to wash twice, the organic phase was dried over anhydrous sodium sulfate, and the organic solvent was evaporated under reduced pressure, and about 2.7g of the product was crystallized from dichloromethane.
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com