Cell transdifferentiation method
A transdifferentiation and cell technology, applied in the field of cell biology, can solve the problems of decreased cell number and proliferation and differentiation potential, high cell virus infection rate, and low separation efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1. Preparation of lentivirus Lentivirus-ETV2.
[0069] The packaging plasma pCMV-dR8.2dvpr, envelope plasma pCMV-VSV-G, and pCCL-ETV2 were respectively amplified in Stbl3 competent cells (TransStbl3Chemically Competent Cell, Cat.No.CD521, TRANS), and the endotoxin-free plasmid was used to Plasmids were extracted using an extraction kit (Cat. No. DP118-02, TIANGEN) to obtain a total amount of 1 mg each.
[0070] 293T cells (Cat.No.CRL-3216, ATCC) with a cell number less than 10 were used, cultured in a complete medium, in a 100mm cell culture dish, and plasmid transfection was ready after the cells reached 80% growth density.
[0071] Add the three plasmids pCMV-VSV-G, pCMV-dR8.2 and pCCL-ETV2 into 1 mL of normal temperature serum-free medium Opti-MEM according to the mass ratio of 1:2.5:2.5 (or molar ratio 1:1:1) , mix gently to prepare a lentiviral expression vector solution (the concentration of the transfected plasmid in each 100mm cell culture dish is 1 μg / ...
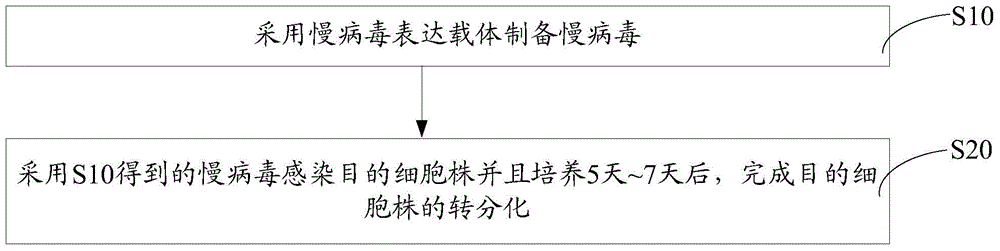
Embodiment 2
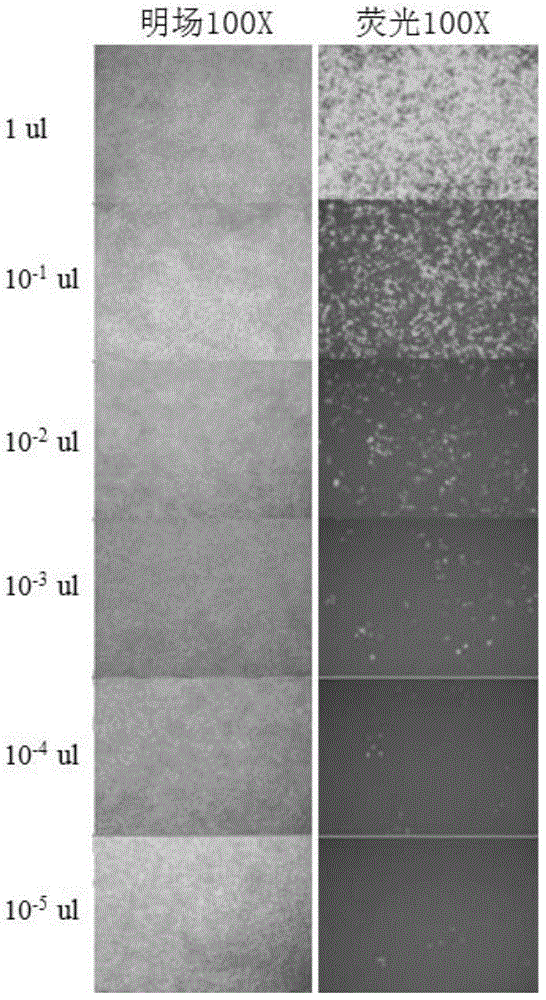
[0082] Embodiment 2, fluorescence counting method is measured the titer of Lentivirus-ETV2
[0083] The day before the measurement, plate the 293T cells required for titer determination, add 5000 cells to each well of a 96-well cell culture plate, and the volume is 100 μL.
[0084] Before infection, perform a 10-fold serial dilution of the lentivirus sample to be detected. The operation method is: prepare 8 sterile 1.5mL centrifuge tubes, add 90 μL of DMEM complete medium to the centrifuge tubes, take 11 μL of the virus sample to be tested and add it to the first tube, mix well, take 11 μL and add it to the second tube. In each tube, continue the same operation until the last tube.
[0085] Select the desired cell wells and aspirate 90 μL of medium. Add 90 μL of the diluted virus solution. Place in the incubator, being careful not to blow up the cells. During the culture process, the medium was replaced appropriately to maintain the normal growth of the cells.
[0086] 37...
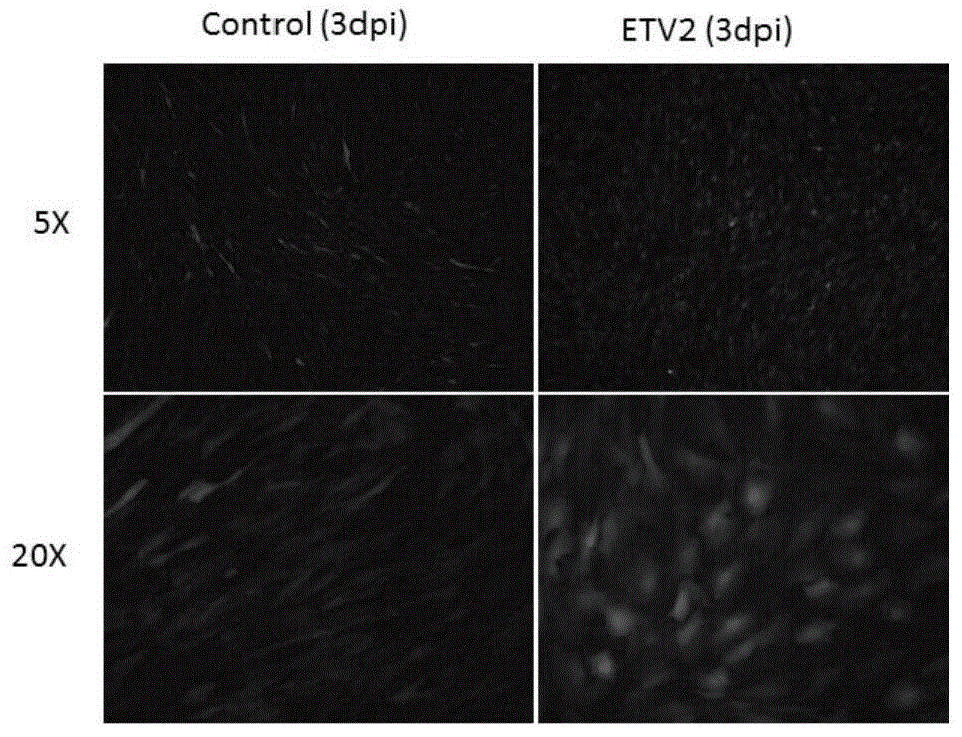
Embodiment 3
[0090] Example 3, Lentivirus-ETV2 infection target cell line
[0091] The target cell line used in this example is human myoblasts (HSKMM, Cat. No. 3520, Lot. No. 2152, ScienCell).
[0092] One day before infection, human myoblasts were plated in a 12-well cell culture plate at a density of 2×10 4 cells / well.
[0093] On the first day of infection, re-digest the cells in similarly treated cell culture wells or dishes, and count the number of cells.
[0094] Heat the complete medium (DMEM medium + 10% FBS) in a 37°C water bath.
[0095] According to the final concentration of 8 μg / μL, polybrene (Hexadimethrine bromide, Cat. No. H9268, Sigma) was added into the complete medium at 37°C, followed by adding lentivirus to obtain the infection medium.
[0096] Remove the medium in the 12-well cell culture plate, and add 400 μL of infection medium according to the ratio of the number of lentivirus to the target cell line at 2TU / cell. Among them, the small-volume infection system h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com