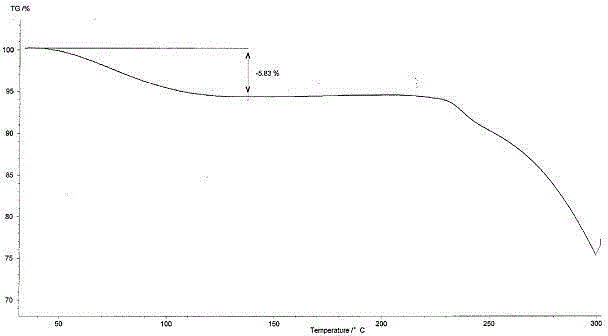
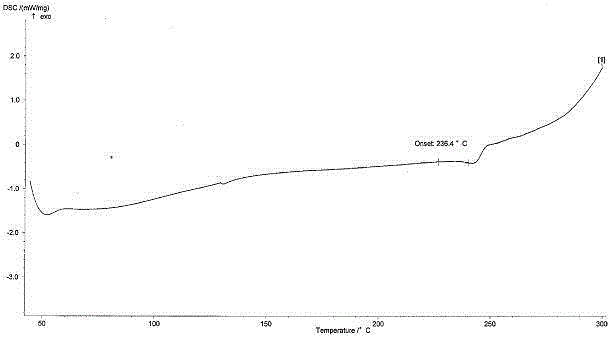
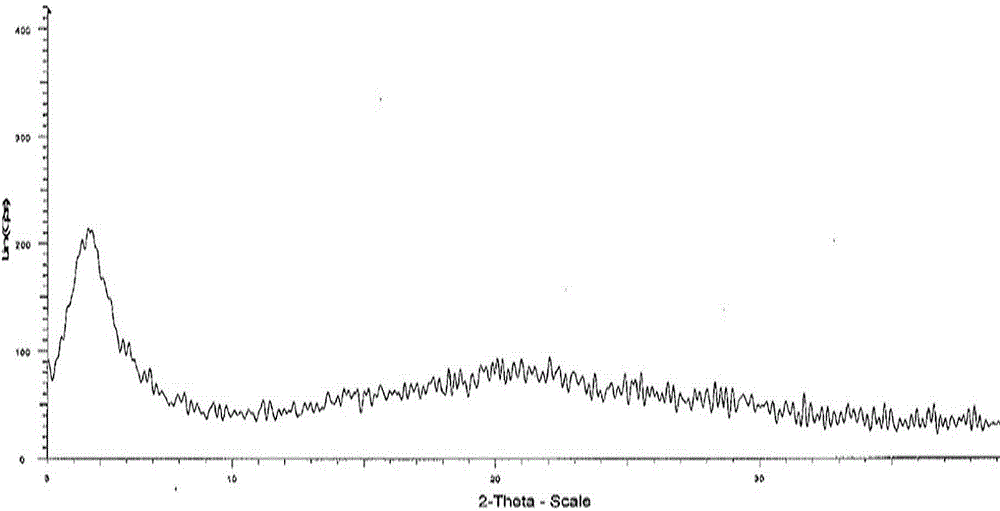
Angiotensin receptor antagonist as well as NEP inhibitor drug crystal form and preparation thereof
A crystal form and solvent technology, applied in the field of angiotensin receptor antagonists and NEP inhibitor drug crystal forms and their preparation, can solve the problems of low solubility and low bioavailability of preparations, and achieve the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Preparation of Amorphous Crystal Form of Trisodium Valsartan ((2R,4S)-5-biphenyl-4-yl-5-(3-carboxy-propionylamino)-2-methyl-valeric acid ethyl ester)
[0018] Add 60mL methanol to a 100 mL three-necked flask, stir magnetically, add 15.0 g valsartan ((2R,4S)-5-biphenyl-4-yl-5-(3-carboxy-propionylamino)-2-methyl -Ethyl valerate) trisodium, (control the temperature throughout the process to T<35°C), stir to dissolve the solid, continue to stir for 30 minutes, evaporate the solvent under negative pressure (<-0.1 Mpa) below 35°C, and obtain the target The crystalline product is 15.0 g, yield: 100%.
Embodiment 2
[0020] Preparation of Amorphous Crystal Form of Trisodium Valsartan ((2R,4S)-5-biphenyl-4-yl-5-(3-carboxy-propionylamino)-2-methyl-valeric acid ethyl ester)
[0021] Add 50 mL of isopropanol to a 100 mL three-necked flask, stir magnetically, add 10 g of valsartan ((2R,4S)-5-biphenyl-4-yl-5-(3-carboxy-propionylamino)-2 -Methyl-valeric acid ethyl ester) trisodium, (control temperature throughout the process to T<35°C), stir to dissolve the solid, continue to stir for 30min, evaporate the solvent under negative pressure (<-0.1 Mpa) below 35°C , to obtain 10 g of the target crystal form product, yield: 100%.
Embodiment 3
[0023] Preparation of Amorphous Crystal Form of Trisodium Valsartan ((2R,4S)-5-biphenyl-4-yl-5-(3-carboxy-propionylamino)-2-methyl-valeric acid ethyl ester)
[0024] Add 25 mL tetrahydrofuran into a 50 mL three-necked flask, stir magnetically, and add 2 g valsartan ((2R,4S)-5-biphenyl-4-yl-5-(3-carboxy-propionylamino)-2-methyl Base-ethyl pentanoate) trisodium, (the temperature is controlled to T<35°C during the whole process), stir to dissolve the solid, continue to stir for 30 minutes, and evaporate the solvent under negative pressure (<-0.1 Mpa) below 35°C. Add 20 mL of acetonitrile to the obtained solid, stir to disperse, filter, and dry under negative pressure (<-0.1 Mpa) at 35±2°C for 24 hours to obtain 1.9 g of the target crystal form product with a yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com