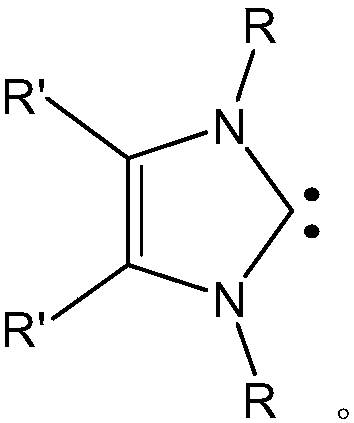
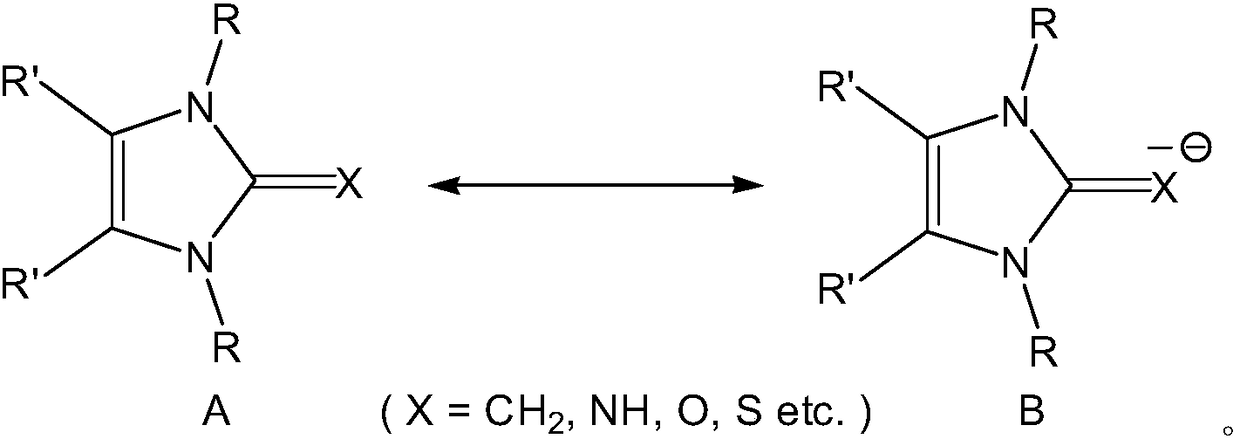
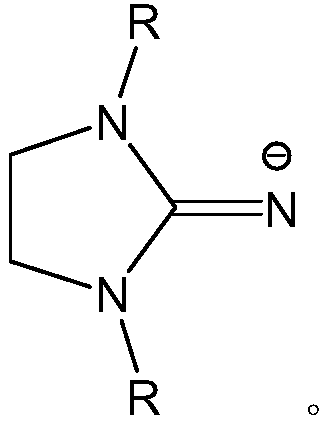
Phenoxy 2-imino imidazolidine metal complex and its preparation method and application
A technology of iminoimidazolidine and metal complexes, which is applied in the direction of palladium organic compounds, nickel organic compounds, chemical instruments and methods, etc., can solve the problem of less late transition metals, achieve less catalyst consumption and good stability of the polymerization system , The effect of convenient synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A preparation method of phenoxy 2-imine imidazolidine nickel complex, comprising the steps of:
[0047] 1) Add oxalyl chloride dropwise to a chloroform solution of 1,3-dimethylimidazolidinone at a concentration of 0.3mmol / mL at 20°C under nitrogen protection. Oxalyl chloride and 1,3-dimethyl The molar ratio of imidazolinone is 4:1, the solvent is removed after heating at reflux at 62°C for 20 hours, and clomethazolium hydrochloride is obtained after washing with anhydrous ether;
[0048] 2) Add triethylamine to the acetonitrile solution of the amino compound with a concentration of 0.5mmol / mL, the molar ratio of triethylamine to the amino compound is 2:1, and then add clomethazole with a concentration of 0.5mmol / mL dropwise Acetonitrile solution of hydrochloride, the molar ratio of clomethazol hydrochloride to amino-containing compound is 1:1, heat and reflux at 82°C for 3h, then add aqueous sodium hydroxide solution with a concentration of 2.0mmol / mL, sodium hydroxide ...
Embodiment 2
[0056] A kind of preparation method of phenoxy 2-imine imidazolidine palladium complex, comprises the steps:
[0057] 1) Add oxalyl chloride dropwise to 0.4mmol / mL 1,3-dimethylimidazolidinone in chloroform solution at 22°C under nitrogen protection. Oxalyl chloride and 1,3-dimethyl The molar ratio of imidazolinone is 4:1, the solvent is removed after heating at reflux at 68°C for 22 hours, and clomethazolium hydrochloride is obtained after washing with anhydrous ether;
[0058] 2) Add triethylamine to the acetonitrile solution of the amino compound with a concentration of 0.6mmol / mL, the molar ratio of triethylamine to the amino compound is 3:1, and then add clomethazole with a concentration of 0.6mmol / mL dropwise Acetonitrile solution of hydrochloride, the molar ratio of clomethazol hydrochloride to amino-containing compound is 1:1, heat and reflux at 83°C for 4h, then add aqueous sodium hydroxide solution with a concentration of 2.0mmol / mL, sodium hydroxide and methyl chlori...
Embodiment 3
[0066] A preparation method of phenoxy 2-imine imidazolidine nickel complex, comprising the steps of:
[0067] 1) Add oxalyl chloride dropwise to 0.5mmol / mL 1,3-dimethylimidazolinone in chloroform solution at 24°C under nitrogen protection, oxalyl chloride and 1,3-dimethyl The molar ratio of imidazolinone is 5:1, the solvent is removed after heating at reflux at 70°C for 23 hours, and clomethazolium hydrochloride is obtained after washing with anhydrous ether;
[0068] 2) Add triethylamine to the acetonitrile solution of the amino compound with a concentration of 0.6mmol / mL, the molar ratio of triethylamine to the amino compound is 4:1, and then add clomethazole with a concentration of 0.6mmol / mL dropwise Acetonitrile solution of hydrochloride, the molar ratio of clomethazol hydrochloride to amino-containing compound is 1:1, heat and reflux at 85°C for 5h, then add aqueous sodium hydroxide solution with a concentration of 2.5mmol / mL, sodium hydroxide and methyl chloride The m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap