A kind of active peptide and application of promoting the immune effect of h9n2 avian influenza vaccine
A technology of immune effects and active peptides, applied in the field of active peptides, can solve the problems of influenza viruses that are more difficult and cannot cope with different virus subtypes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Isolation and identification of BP4
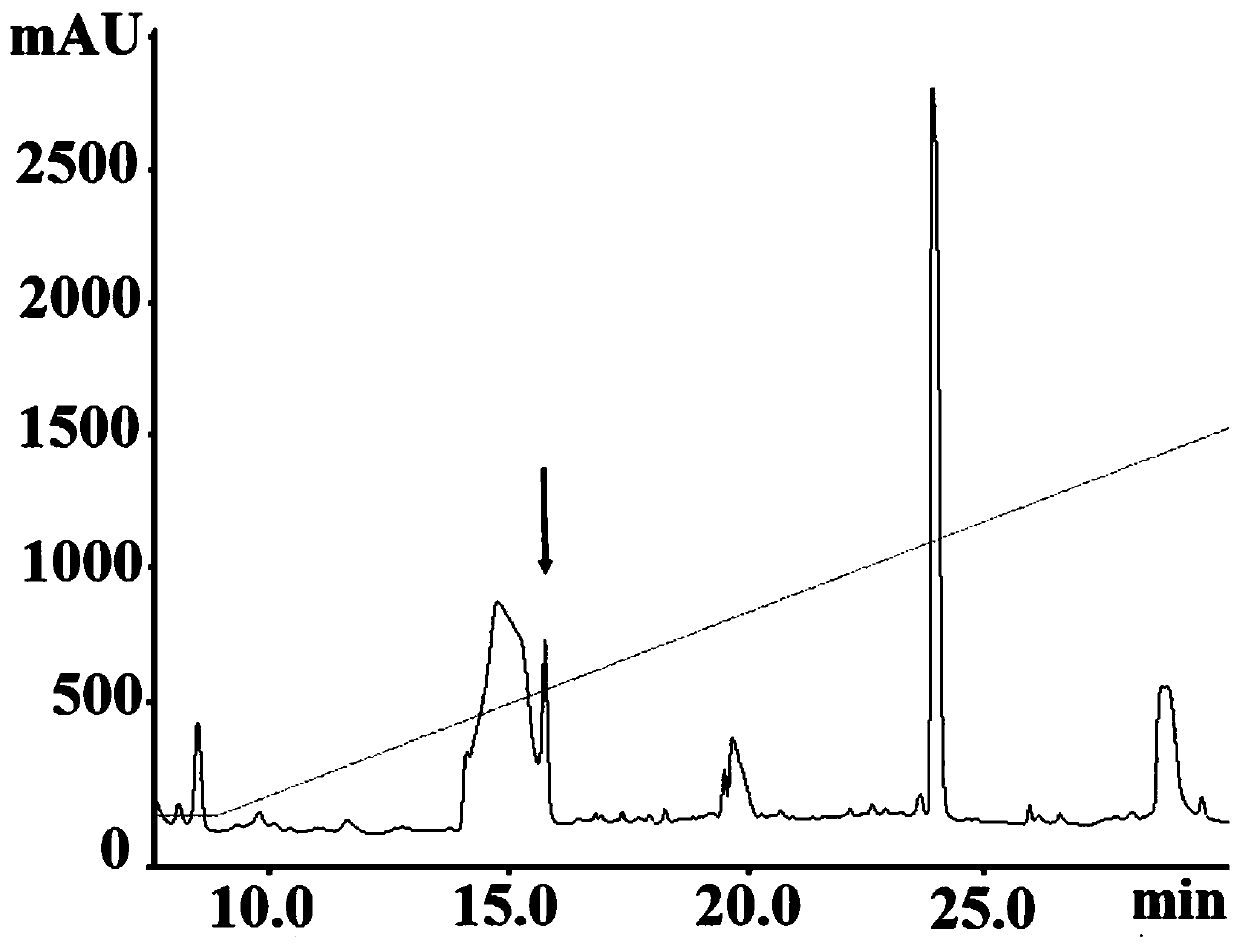
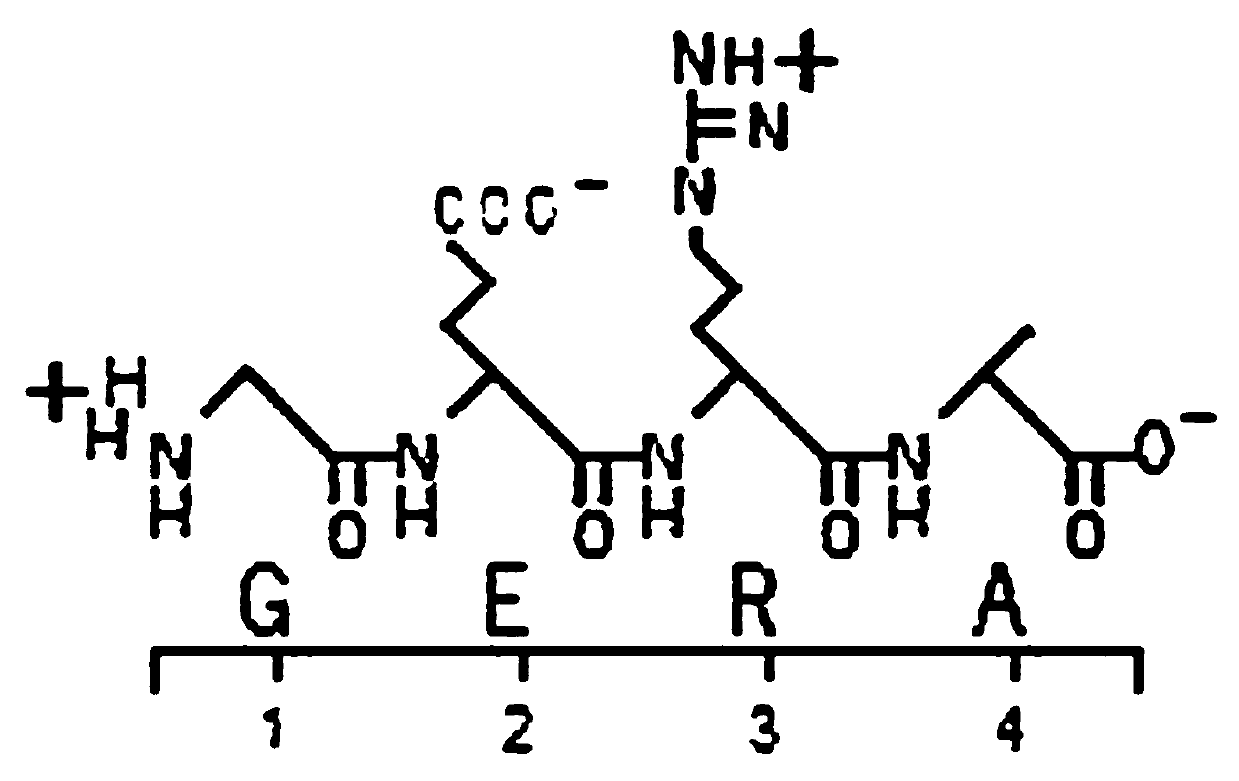
[0026] Take 500 grams of the bursa of a healthy 4-6 week-old chicken (AA broiler, farm affiliated to Shanghai Academy of Agricultural Sciences), crush it with 1000 ml of normal saline, freeze and thaw three times, and centrifuge at 14000 g for 60 minutes at low temperature. The supernatant was ultrafiltered at 1000 Da and freeze-dried. After the lyophilized sample was diluted, it was filtered with a 0.22um filter membrane, and then the reverse high performance liquid phase purification analysis was performed, and the activity peak with a retention time of 15.72min was harvested (see figure 1 ). The harvested eluate was analyzed by MALDI-TOF-MS, the molecular weight was 431.213, the amino acid sequence GERA (see figure 2 ).
Embodiment 2
[0028] 1. Synthetic BP4
[0029] Entrust commercial peptide synthesis company according to COOH-GERA-NH2 amino acid
[0030] The sequence (SEQ ID NO: 1) synthesizes the polypeptide, and the purity is required to be ≧97%.
[0031] 2. Vaccines
[0032] Inactivated bird flu vaccine (H9N2 subtype F strain, purchased from Nanjing Meria Animal Health Co., Ltd.)
[0033] 3. Grouping of experimental animals
[0034] 50 BALB / C mice were randomly divided into five groups, 10 in each group: (1) avian influenza inactivated vaccine immunization group (0.2ml of each immune inactivated vaccine); (2~4) inactivated vaccine+BP4 group (BP4 concentrations were 10, 50, 250ug / mL, and the immunization dose was 0.2ml); PBS control group (each immunized with 0.2ml PBS), immunized twice with an interval of 2 weeks.
[0035] 4. Sample Collection
[0036] Two weeks after the second immunization, blood was collected from the orbit every week, and the serum was separated by centrifugation at 8,000×g f...
Embodiment 3
[0048] 1. Synthetic BP4
[0049] Entrust a commercial peptide synthesis company according to COOH-GERA-NH2 amino acid, the purity is ≧97%.
[0050] 2. Vaccines
[0051] Inactivated bird flu vaccine (H9N2 subtype F strain, purchased from Nanjing Meria Animal Health Co., Ltd.)
[0052] 3. Grouping of experimental animals
[0053]45 75-day-old chickens (Baihang chickens, purchased from Qinglongshan Farm) were randomly divided into three groups, 15 in each group: (1) Bird flu inactivated vaccine immunization group (each immunized inactivated vaccine 0.5ml) (2) Inactivated vaccine+BP4 group (BP4 concentration is 50ug / mL, immunization dose is 0.5ml); PBS control group (each immunized with 0.5ml PBS).
[0054] 4. Sample Collection
[0055] On the 2nd and 4th weeks after immunization, anticoagulant blood was collected from each chicken wing, and the serum was separated by centrifugation at 8000×g for 10 minutes. Antibody titers and cytokine expression levels were measured, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com