A kind of cell membrane targeted mg2+ fluorescent probe and its preparation method and application
A technology of fluorescent probes and cell membranes, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve problems such as difficulty in judging concentration changes, and achieve the effect of enhanced fluorescence intensity and dynamic reversible response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
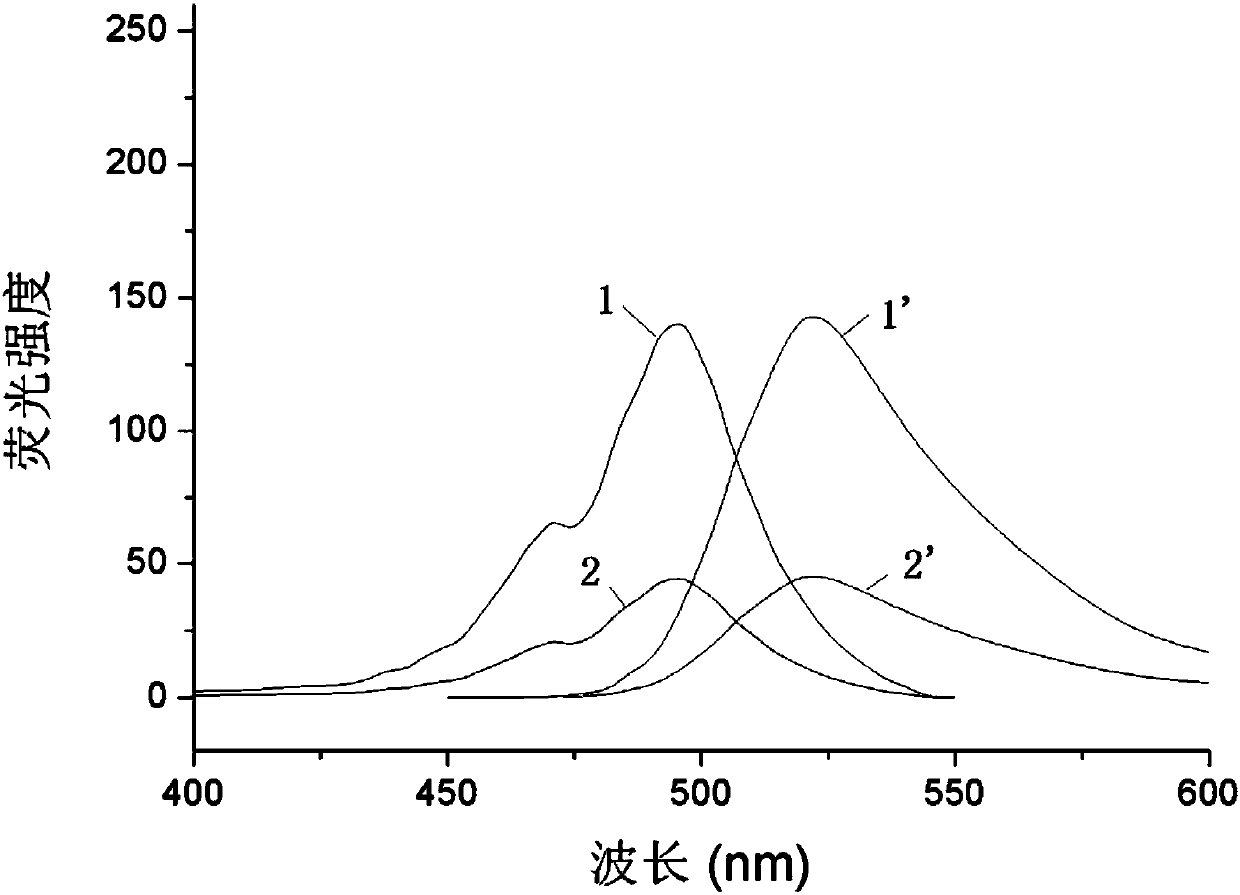
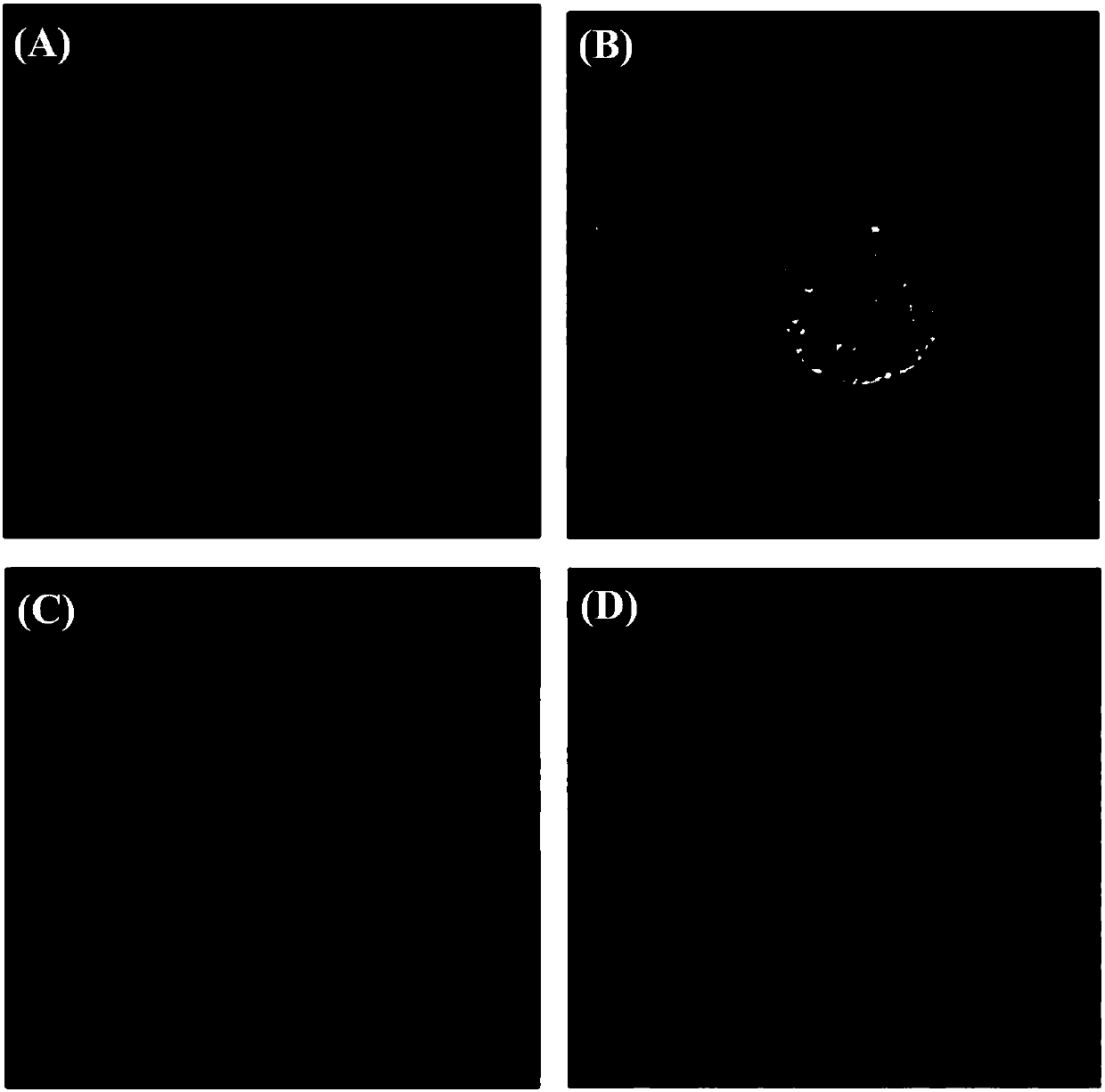
Image
Examples
Embodiment 1
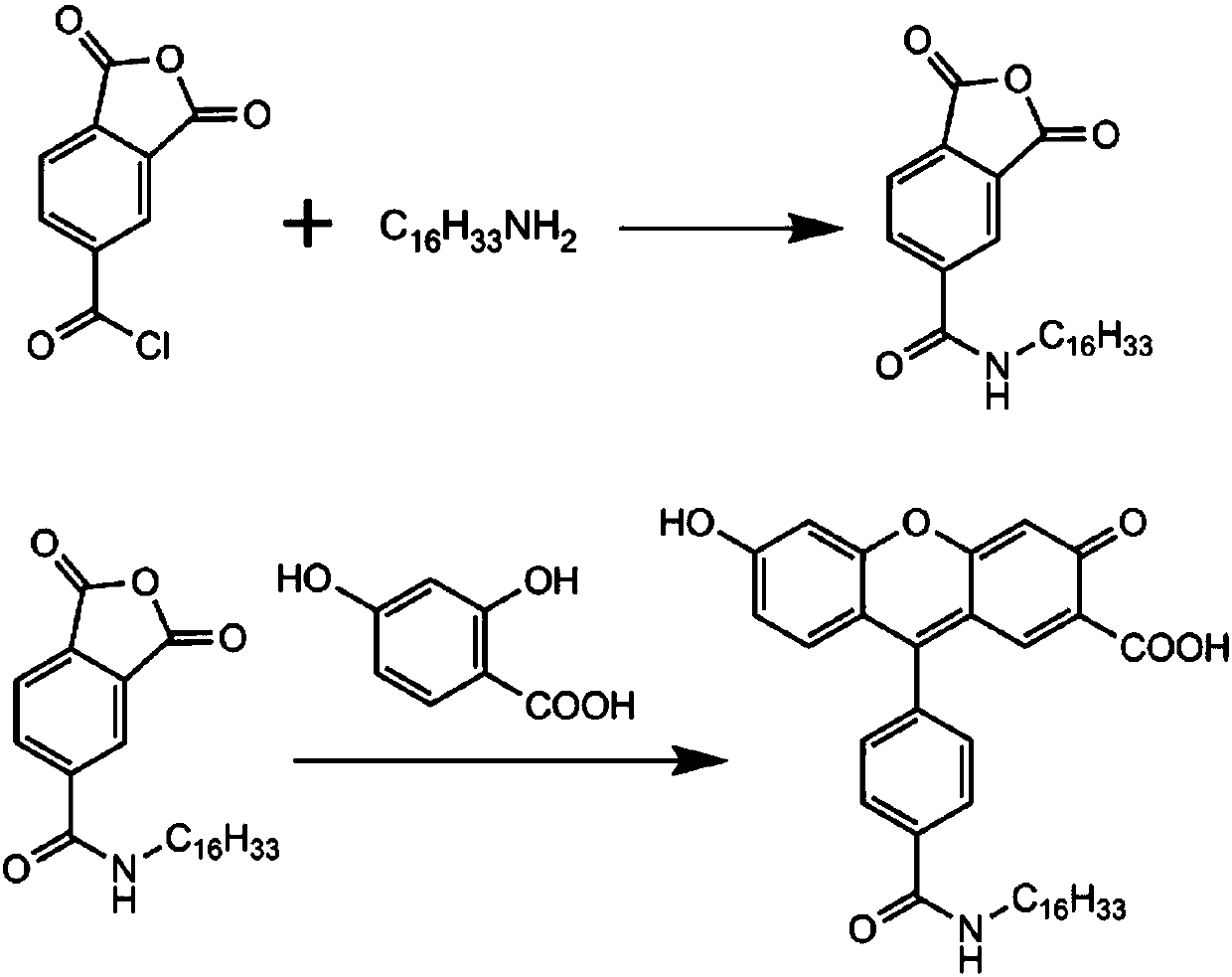
[0030] Dissolve 2.10 g of trimellitic anhydride (0.1 mol) in 50 mL of dichloromethane, add dropwise a solution of 2.4 g of hexadecylamine (0.1 mol) in 50 mL of dichloromethane at room temperature, stir the mixture at room temperature for 1 hour, and remove the solvent under reduced pressure. 4.1 g of crude hexadecyl trimellitic anhydride was obtained, which was directly used in the next reaction without further purification.
[0031] Add 416 mg hexadecyl trimellitic anhydride (1 mmol) and 154 mg 2,4-dihydroxybenzoic acid (1 mmol) to 20 mL methanesulfonic acid, and keep warm at 85° C. for 3 hours. Cool to room temperature, neutralize methanesulfonic acid with 5mol / L NaOH solution, and then acidify with 1mol / L hydrochloric acid, a large amount of orange-yellow precipitates are formed. The solid was collected by filtration, separated and purified by column chromatography to obtain 65 mg of 1-carboxy-5-formylhexadecanylfluorescein. 1 H NMR: 0.87-0.90(t,3H),1.28(s,28H),3.40-3.46(m...
Embodiment 2
[0033] Dissolve 2.10 g of trimellitic anhydride (0.1 mol) in 50 mL of dichloromethane, add dropwise a solution of 2.4 g of hexadecylamine (0.1 mol) in 50 mL of dichloromethane at room temperature, stir the mixture at room temperature for 3 hours, and remove the solvent under reduced pressure. The crude product of hexadecyl trimellitic anhydride was directly used in the next reaction without further purification.
[0034] Hexadecyl trimellitic anhydride (1 mmol) and 154 mg of 2,4-dihydroxybenzoic acid (1 mmol) were added to 20 mL of methanesulfonic acid, and kept at 40° C. for 24 hours. Cool to room temperature, neutralize methanesulfonic acid with 5mol / L NaOH solution, and then acidify with 1mol / L hydrochloric acid, a large amount of orange-yellow precipitates are formed. The solid was collected by filtration, separated and purified by column chromatography to obtain 1-carboxy-5-formylhexadecanylfluorescein.
Embodiment 3
[0036] Dissolve 2.10 g of trimellitic anhydride (0.1 mol) in 50 mL of dichloromethane, add dropwise a solution of 2.4 g of hexadecylamine (0.1 mol) in 50 mL of dichloromethane at room temperature, stir the mixture at room temperature for 5 hours, and remove the solvent under reduced pressure. The crude product of hexadecyl trimellitic anhydride was directly used in the next reaction without further purification.
[0037] Hexadecyl trimellitic anhydride (1 mmol) and 154 mg of 2,4-dihydroxybenzoic acid (1 mmol) were added to 20 mL of methanesulfonic acid, and kept at 90° C. for 1 hour. Cool to room temperature, neutralize methanesulfonic acid with 5mol / L NaOH solution, and then acidify with 1mol / L hydrochloric acid, a large amount of orange-yellow precipitates are formed. The solid was collected by filtration, separated and purified by column chromatography to obtain 1-carboxy-5-formylhexadecanylfluorescein.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com