Dianthranide-based compounds as well as preparation method and application thereof as high-pressure transducer
A compound and aryl technology, which is applied in the field of fluorescent compounds and their preparation, can solve problems such as poor measurement, expensive detection instruments, and inconvenient convenience, and achieve the effects of wide detection range, wide practical range, and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation of compound 1, its reaction scheme is as follows:
[0053]
[0054] First add 9-anthrone (5g, 26mmol), zinc powder (8.5g, 129mmol), zinc chloride (7g, 56mmol) into the three-necked flask, then add a certain proportion of tetrahydrofuran and water mixed solvent 100ml, add magnetic After stirring at room temperature for more than 8 hours, extract with dichloromethane, dry with anhydrous magnesium sulfate, filter, spin dry, and obtain 4.8 g of a yellow solid after drying, then add the yellow solid into a three-necked flask, and add a small amount of p-toluenesulfonic acid ( PTS), use toluene as a solvent, reflux until the solvent becomes clear, extract with solvent dichloromethane and dry with desiccant anhydrous magnesium sulfate, filter, spin dry, and obtain 4.3g of white crystalline solid by silica gel column purification , yield 93%.
[0055] Preparation of compound 2, its reaction scheme is as follows:
[0056]
[0057] Dissolve 3g of compound 1 (8...
Embodiment 2
[0062] Compound PAAP is prepared, wherein the reaction scheme is as follows:
[0063]
[0064] Wherein the synthetic method of compound 1 sees embodiment 1
[0065] Preparation of Compound 3: Compound 1 (1g, 2.8mmoL) was added to a three-neck flask, dissolved in 100mL of chloroform solvent, slowly added dropwise to 50mL of a solution containing N-bromosuccinimide (1.2g, 6.8mmoL), and stirred at room temperature 3-8h. It was extracted with dichloromethane as a solvent, dried with anhydrous magnesium sulfate as a desiccant, filtered, spin-dried, and purified with petroleum ether through a silica gel column to obtain 1.35 g of a solid with a yield of 95%.
[0066] Preparation of compound PAAP: Compound 3 (1g, 1.96mmoL) was added to a three-necked flask, vacuumed and replaced with nitrogen three times, dissolved in 100mL of dry and refluxed tetrahydrofuran, bathed in acetone on dry ice, and 3mL of n-butyllithium was slowly added dropwise after about half an hour. After low-te...
Embodiment 3
[0068] Preparation contains the macromolecular film of compound DAAD among the embodiment 1, and preparation method is that compound DAAD and macromolecular material polymethyl methacrylate are dissolved in methylene chloride solution, wherein the mass ratio of compound DAAD and macromolecular material is 1: 200, pour it into a clean petri dish after fully stirring, and put it in a 45°C oven to dry.
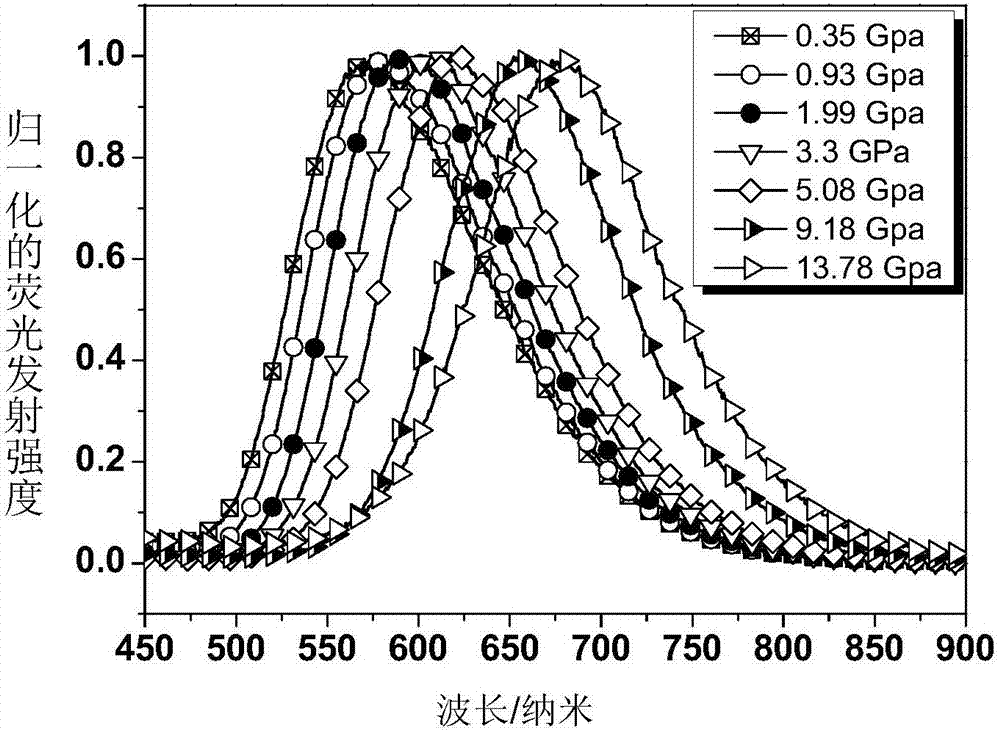
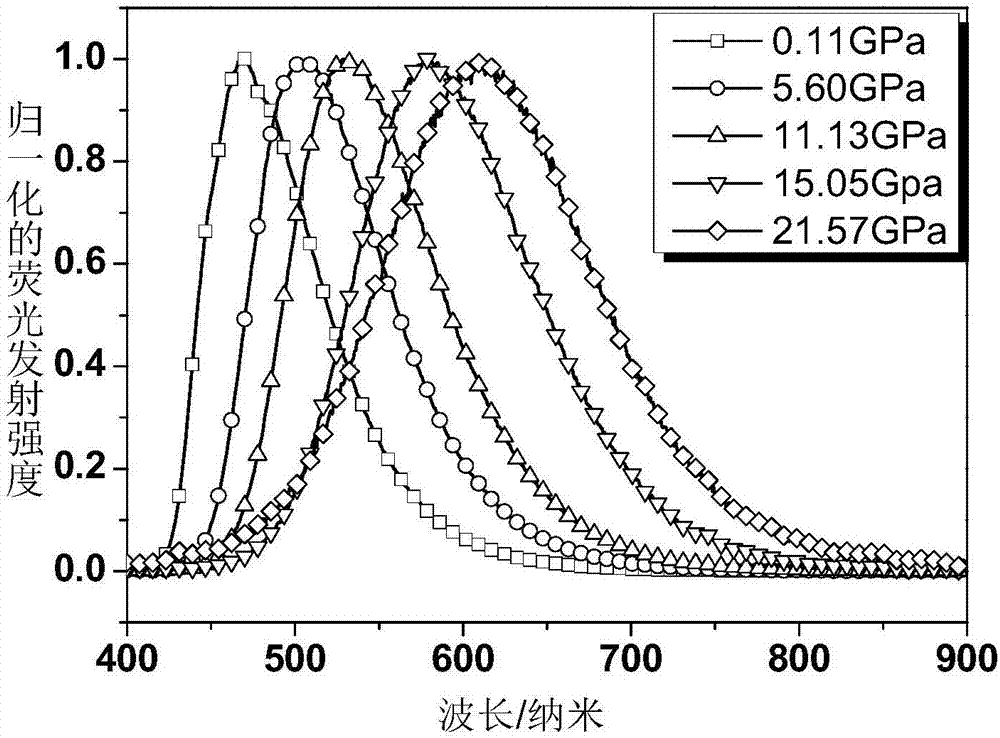
[0069] figure 1 For the fluorescence spectrum of the polymer film containing compound DAAD prepared in Example 3 under different pressures, it can be seen from the figure that as the pressure increases, the wavelength of fluorescence emission has been red-shifted from about 572nm to about 677nm, The corresponding fluorescent color changes gradually from green to yellow to orange and finally to deep red.
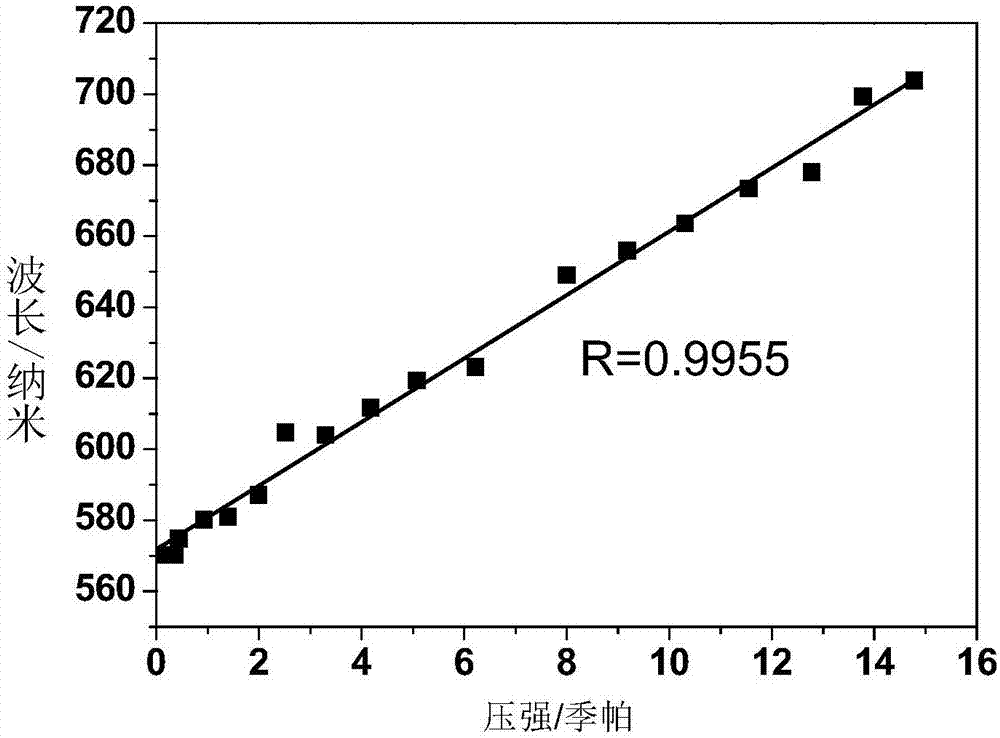
[0070] figure 2 The linear curve between the maximum fluorescence wavelength and each pressure of the polymer film containing compound DAAD prepared in embodiment 3 under dif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com