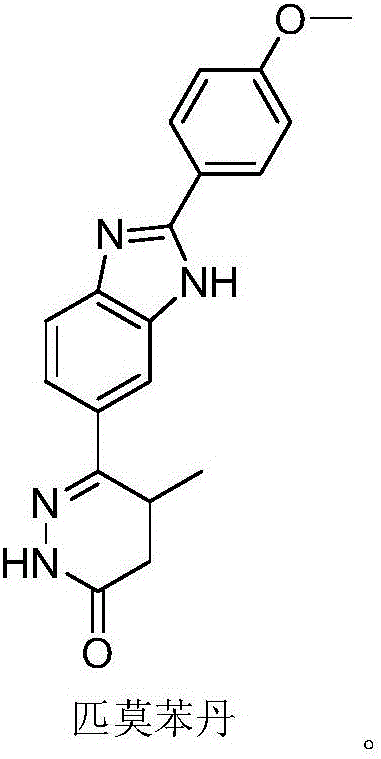
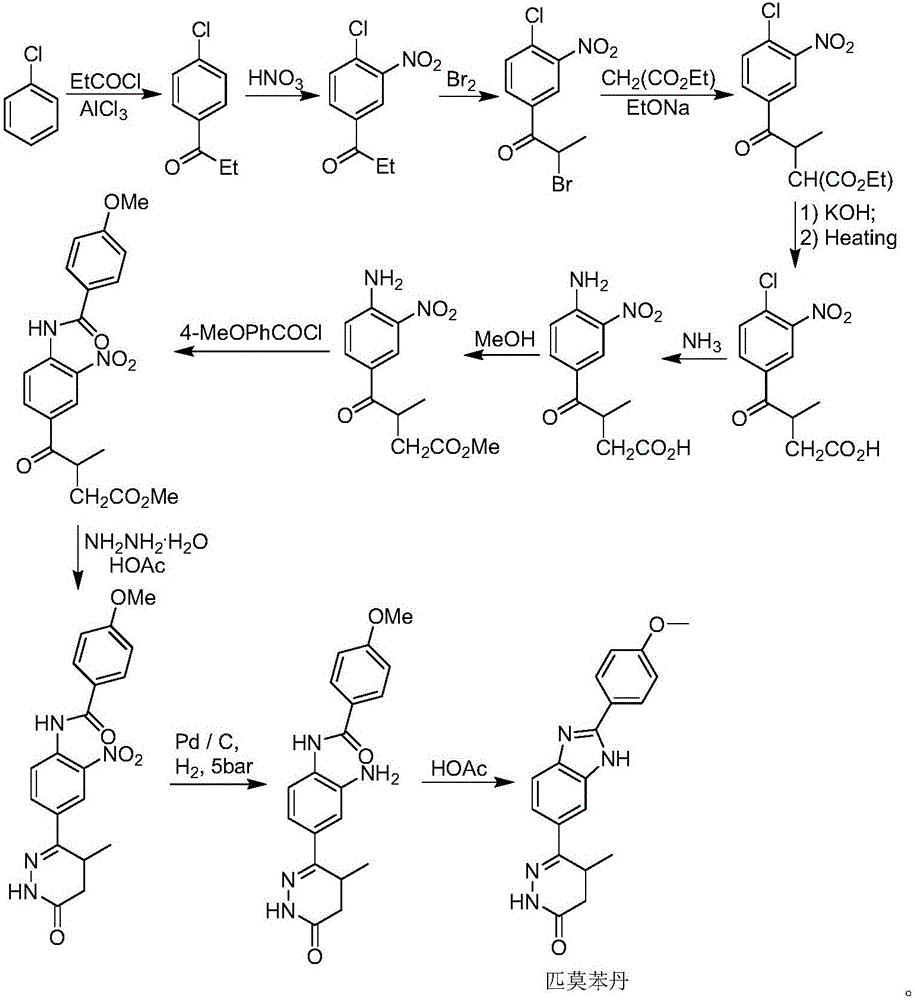
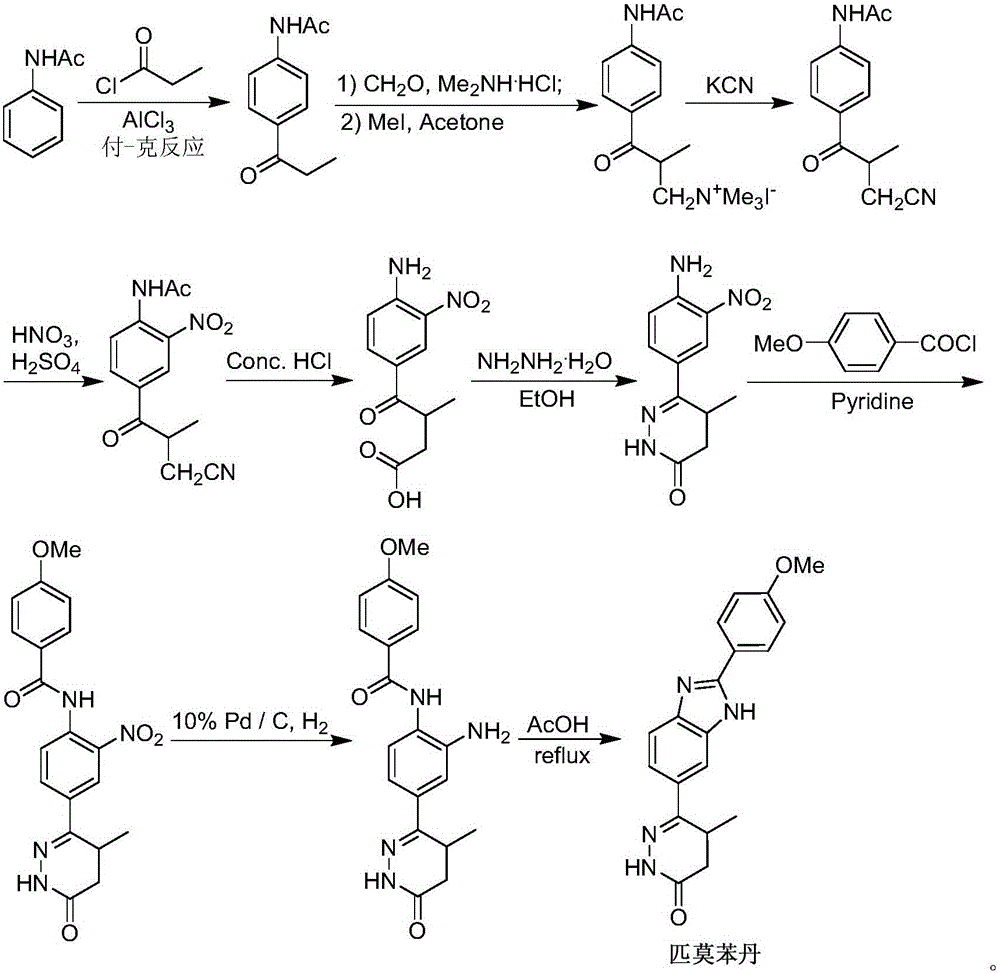
Preparation of 6-(3,4-diamino phenyl)-5-methyl-4,5-dihydropyridazine-3(2H)-ketone key intermediate of 2-(4-methoxyphenyl)-5(6)-(5-methyl-3-oxo-4,5-dihydro-2H-6-pyridazinyl)benzimidazole
A technology of diaminophenyl and dihydropyridazine, applied in the field of key intermediates of pimobendan, can solve problems such as being unsuitable for industrial scale-up production, and achieve the effects of short process route and mild reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0022] 1. Preparation of 4-(4-acetylamino-3-nitrophenyl)-3-methyl-4-oxobutanoic acid (formula II)
[0023] 140 grams of nitric acid (93mL) and 20 grams of sulfuric acid (11mL) were mixed and placed in a reaction flask to cool below -5°C, and 4-(4-acetylaminophenyl)-3-methyl-4-oxobutyric acid (Formula I) (31g, 0.124mol) was slowly added in batches, and the temperature of the system was controlled at -5°C during the addition process (3-5 grams each time, with an interval of about 15 minutes). After feeding, the system was stirred at about 0°C for 4 hours, and then the reaction solution was slowly added to the solution made of 300mL water and 90g NaCl (cooled to about 0°C in advance), and fully stirred after adding. The system was slowly raised to room temperature, and then the reaction solution was extracted with ethyl acetate (300 mL), and the organic phase was separated. The aqueous phase was further extracted with ethyl acetate (360 mL). Combine the organic phases, wash the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com