A kind of synthetic method of high molecular weight polycaprolactone
A synthesis method and polycaprolactone technology are applied in the field of catalytic synthesis of high molecular weight polycaprolactone, which can solve problems such as difficult commercial production and achieve the effect of stable and reliable operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
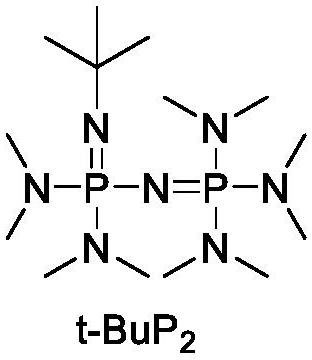
[0028] t-BuP 2 (25 μmol, 1 equiv.) and phenylpropanol (25 μmol, 1 equiv.) were mixed into a polymerization tube with 5 mL of dichloromethane, and stirred evenly. Then ε-caprolactone (2.5mmol, 100equiv.), ZnCl 3 (37.5 μmol, 1.5 equiv) was added and mixed into the reaction system to start the polymerization reaction, the polymerization reaction was carried out at room temperature, and the whole system was under the protection of argon. used during the reaction 1 H NMR was used to detect the monomer content. When the monomer in the system was completely consumed, an excess of benzoic acid was added, and then triethylamine was added to terminate the reaction. The dichloromethane was spun off with a rotary evaporator to obtain the crude product. Use toluene to separate out the complexes and metal salts in the crude product, filter off the solid impurities, and then spin off the toluene. Finally, the product was redissolved in a small amount of CH 2 Cl 2 Then, the mixed liquid...
Embodiment 2
[0030] t-BuP 2 (25 μmol, 1 equiv.) and butanol (25 μmol, 1 equiv.) were mixed into a polymerization tube with 4 mL of dichloromethane, and stirred evenly. Then ε-caprolactone (1.75mmol, 70equiv.), AlCl 3 (30 μmol, 1.2 equiv.) was added and mixed into the reaction system to start the polymerization reaction, the polymerization reaction was carried out at room temperature, and the whole system was under the protection of argon. used during the reaction 1 H NMR was used to detect the monomer content. When the monomer in the system was completely consumed, an excess of benzoic acid was added, and then triethylamine was added to terminate the reaction. The dichloromethane was spun off with a rotary evaporator to obtain the crude product. Use toluene to separate out the complexes and metal salts in the crude product, filter off the solid impurities, and then spin off the toluene. Finally, the product was dissolved in a small amount of CH 2 Cl 2 Then, the mixed liquid was added...
Embodiment 3
[0032] t-BuP 2 (25 μmol, 1 equiv.) and phenylpropanol (25 μmol, 1 equiv.) were mixed into a polymerization tube with 3 mL of dichloromethane, and stirred evenly. Then ε-caprolactone (3.75mmol, 150equiv.), FeCl 3 (42.5 μmol, 1.7 equiv.) was added and mixed into the reaction system to start the polymerization reaction, the polymerization reaction was carried out at room temperature, and the whole system was under the protection of argon. used during the reaction 1 H NMR was used to detect the monomer content. When the monomer in the system was completely consumed, an excess of benzoic acid was added, and then triethylamine was added to terminate the reaction. The dichloromethane was spun off with a rotary evaporator to obtain the crude product. Use toluene to separate out the complexes and metal salts in the crude product, filter off the solid impurities, and then spin off the toluene. Finally, the product was dissolved in a small amount of CH 2 Cl 2 Then, the mixed liquid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com