Exposing method for transformation-mode nanomaterial induced mammalian cell malignant transformation
A nanomaterial, mammalian technology, applied in animal cells, vertebrate cells, embryonic cells, etc., can solve problems such as inability to accurately reflect real toxicity, and achieve the effect of being suitable for large-scale promotion, simple method and reliable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The present invention provides a kind of technical scheme step as follows:
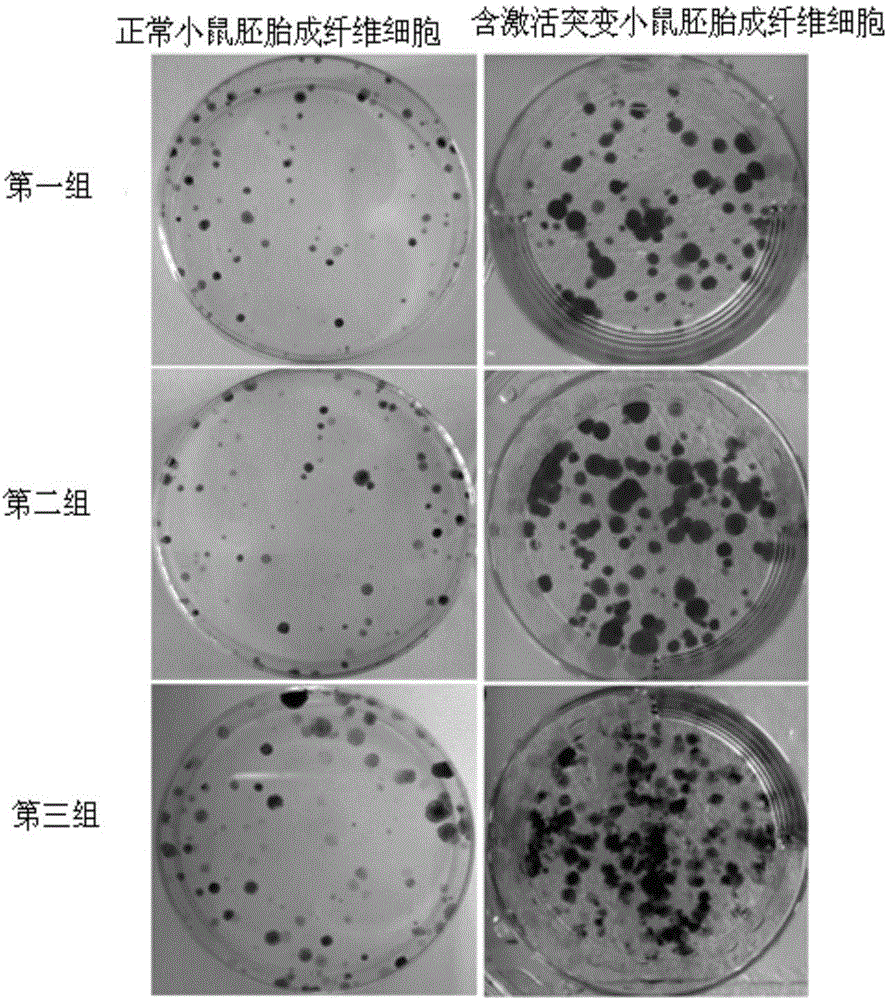
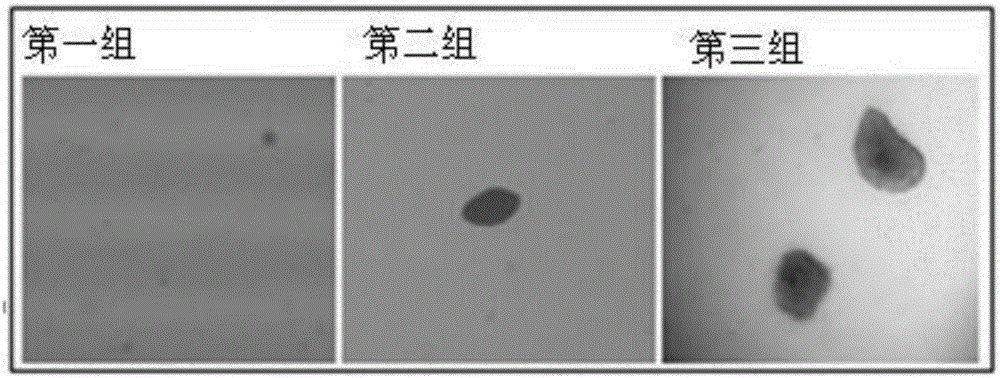
[0032] Step 1: culture normal mouse embryonic fibroblasts and mouse embryonic fibroblasts containing activating mutations in DMEM medium containing fetal bovine serum in an incubator;
[0033] Step 2: Take three dishes of normal mouse embryonic fibroblasts and mouse embryonic fibroblasts containing activating mutations in the logarithmic growth phase and 80% fullness after the treatment in step 1 and divide them into three groups. The group included a dish of normal mouse embryonic fibroblasts and a dish of mouse embryonic fibroblasts containing activating mutations, and the groups were marked, and the number of plants in each dish was 1×10 5 , the size of the culture dish is 60mm, and each group of cells is digested with trypsin;
[0034] Step 3: After the three groups of cells in step 2 grow to the logarithmic growth phase, discard the culture medium in step 2, add 4 ml of DMEM medium contai...
Embodiment 2
[0047] Proceed as follows:
[0048] Step 1: culture normal mouse embryonic fibroblasts and mouse embryonic fibroblasts containing activating mutations in DMEM medium containing fetal bovine serum in an incubator;
[0049] Step 2: Take three dishes of normal mouse embryonic fibroblasts and mouse embryonic fibroblasts containing activating mutations in the logarithmic growth phase and 80% fullness after the treatment in step 1 and divide them into three groups. The group included a dish of normal mouse embryonic fibroblasts and a dish of mouse embryonic fibroblasts containing activating mutations, and the groups were marked, and the number of plants in each dish was 1×10 5 , the size of the culture dish is 60mm, and each group of cells is digested with trypsin;
[0050] Step 3: After the three groups of cells in step 2 grow to the logarithmic growth phase, discard the culture medium in step 2, add 4 ml of DMEM medium containing 10% volume of fetal bovine serum to the first grou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com