Application of cyclic dinucleotide cgamp in anti-liver metastasis of colorectal cancer
A technology for colorectal cancer and liver metastases, applied in the field of biomedicine, can solve the problem that liver metastases cannot be radically removed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Embodiment 1: Preparation of cGAMP
[0012] cGAMP (cyclized-GMP-AMP) is catalyzed and synthesized by cyclized cGMP-AMP dinucleotide synthetase (cGAS) under activation conditions after binding to DNA according to literature method. The purity is above 98%. (Pingwei Li, et al., Immunity, 2013, 39(6), 1019-1031.)
Embodiment 2
[0013] Example 2: Anti-tumor effect of cGAMP (cyclized-GMP-AMP) The tumor-bearing mouse model was used to detect the inhibitory effect of cGAMP on liver metastasis of colorectal cancer in animals.
[0014] Test drug
[0015] Name: cGAMP
[0016] Appearance: white powder
[0017] Vehicle: normal saline.
[0018] Preparation method: Prepare the solution with the required concentration with physiological saline solution before use.
[0019] Test drug concentration: 1mg / ml, 2mg / ml.
[0020] animal
[0021] Species, strains, gender, body weight, source, certificate of conformity
[0022] C57 mice, male, weighing 16-18 g, 6-8 weeks old, were purchased from Shanghai Slack Experimental Animal Co., Ltd. [Experimental animal quality certificate number: SCXK (Shanghai) 2007-0005].
[0023] Feeding conditions
[0024] All mice were free to forage for food and drink water, and were kept at room temperature (23±2)°C in the Experimental Animal Center of a Military Medical University o...
Embodiment 3
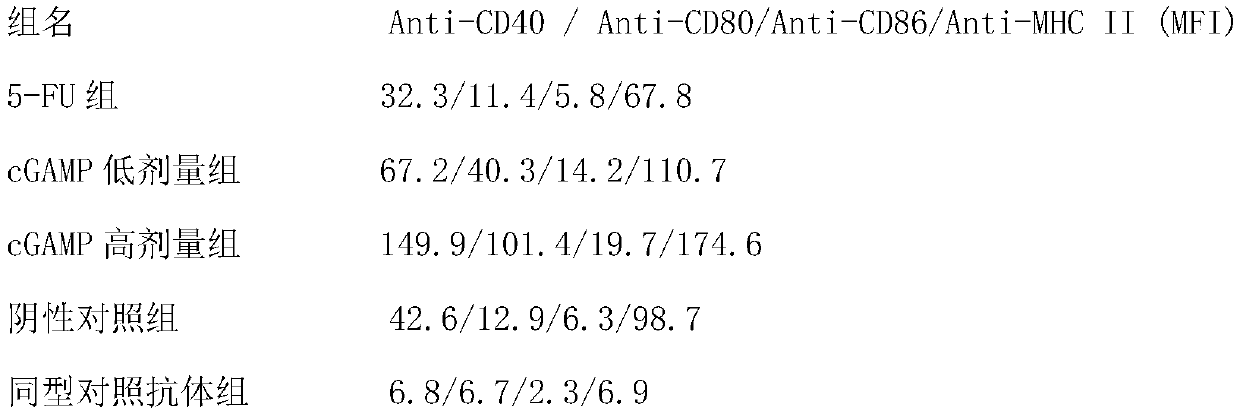
[0049] Embodiment 3: Activation efficiency of mouse dendritic cells (DC cells) tested by flow cytometry
[0050] See Example 2 for mouse breeding and colorectal cancer metastasis model modeling. Anti-CD40, CD80, CD86, MHC II, isotype control antibodies and other flow antibodies were purchased from eBiosciences, CD11c+ antibody magnetic beads were purchased from MiltenyiBiotech, and flow cytometers were purchased from BD. The mice were killed 14 days after the administration, and the spleens of the mice were taken, ground and crushed, and the cells were filtered through a 40 μm pore membrane. Centrifuge at 1000rpm for 10 minutes to separate the immune cells in the spleen, lyse the erythrocytes with erythrocyte lysate until the erythrocytes are completely dissolved, and then centrifuge at 1000rpm for 10 minutes to separate the unlysed immune cells. Separate DC cells with CD11c+ antibody magnetic beads, add anti-CD40, CD80, CD86, MHC II FAC antibodies (diluted with FACS buffer) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com