Specific primer set and detection kit for detecting drug resistance mutation gene of Mycoplasma pneumoniae
A technology for Mycoplasma pneumoniae and drug resistance mutation, which is applied in the directions of microorganism-based methods, microorganisms, recombinant DNA technology, etc., can solve the problem that the upstream primer hypoxanthine insertion site adversely improves the sensitivity, the detection sensitivity of Mycoplasma pneumoniae drug resistance is low, and the sensitivity and The specificity cannot be improved at the same time, so as to achieve the effect of good consistency, high sensitivity and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Establishment of Mycoplasma pneumoniae drug-resistant mutation gene detection method
[0059] 1. Primer Design
[0060] This kit uses the 23S rRNA V region sequence of Mycoplasma pneumoniae as a template to design upstream primers (23NU, 2063MU, 2064MU), and introduces the mismatched base hypoxanthine I at the penultimate 2, 3, and 4 positions of the 3' end of the specific primer 2063MU. , the mismatched base hypoxanthine I was introduced into the penultimate 3, 4, and 5 positions of the 3' end of the specific primer 2064MU, and hypoxanthine was introduced into the penultimate position of the 3' end of its downstream primer (23D) for the first time I, to increase primer specificity. A total of 16 pairs of primers were designed during the development of this kit, and the best primer pair was selected after multiple verifications.
[0061] 2. Standard product construction: construct the wild-type and mutant plasmids containing the target gene locus, perform qu...
Embodiment 2
[0064] Embodiment 2 Preparation and use method of kit of the present invention
[0065] The composition of kit of the present invention:
[0066] 1. The primer sequence group that is used to detect Mycoplasma pneumoniae mutant gene, described primer group is shown in SEQ ID No.1 by wild-type upstream primer sequence, A2063G mutant type upstream primer sequence is shown in SEQ ID No.2, A2064G mutation The sequence of the type upstream primer is shown in SEQ ID No.3, and the sequence of the universal downstream primer is shown in SEQ ID No.4.
[0067] 2. The PCR reaction solution can be a composition known to those skilled in the art. The recommended composition of the present invention is: 25 μl of PCR buffer UltraSYBR Mixture (With ROX), (UltraSYBR Mixture, purchased from Beijing Kangwei Century Biotechnology Co., Ltd., article number: CW2601M) . Nucleic acid-free water 22 μl, template 2 μl.
[0068] The method for using the kit of the present invention:
[0069] 1. Extrac...
Embodiment 3
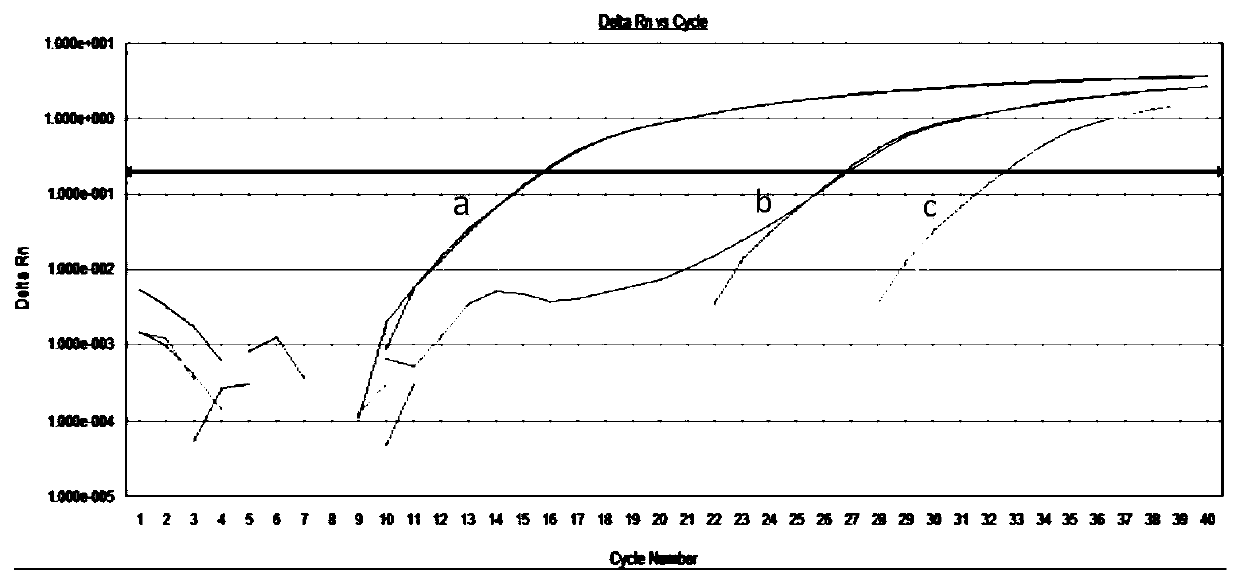
[0095] Embodiment 3 kit sensitivity and specificity verification experiment
[0096] (1) Standard curve drawing
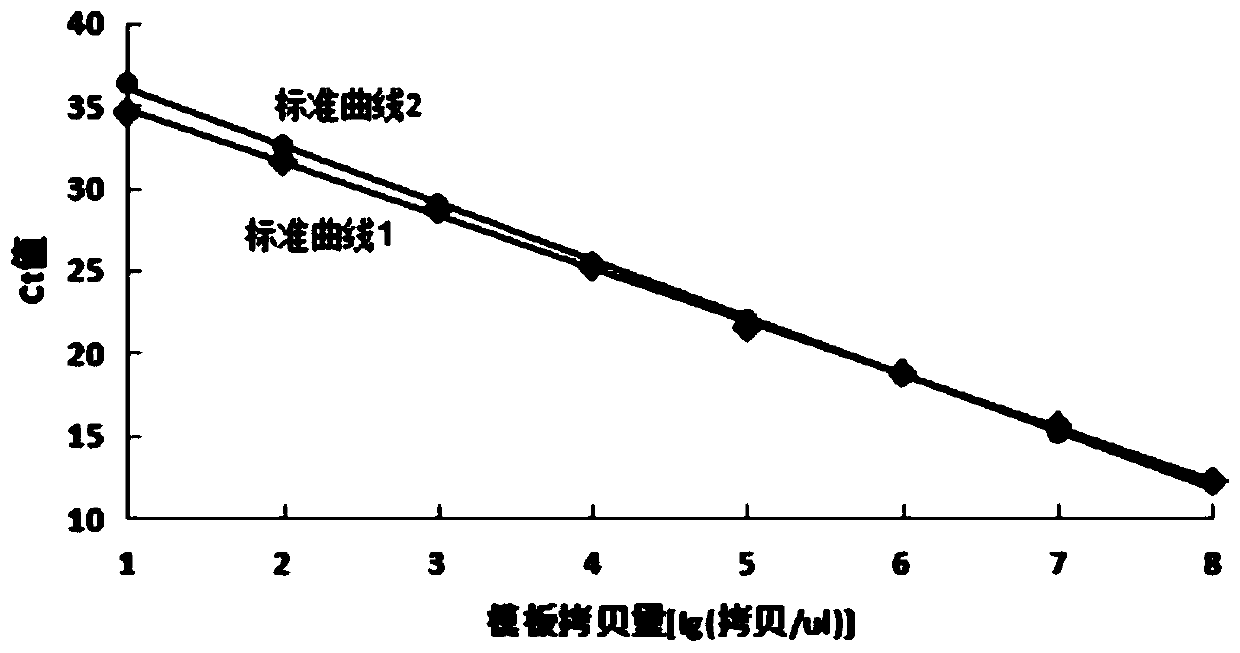
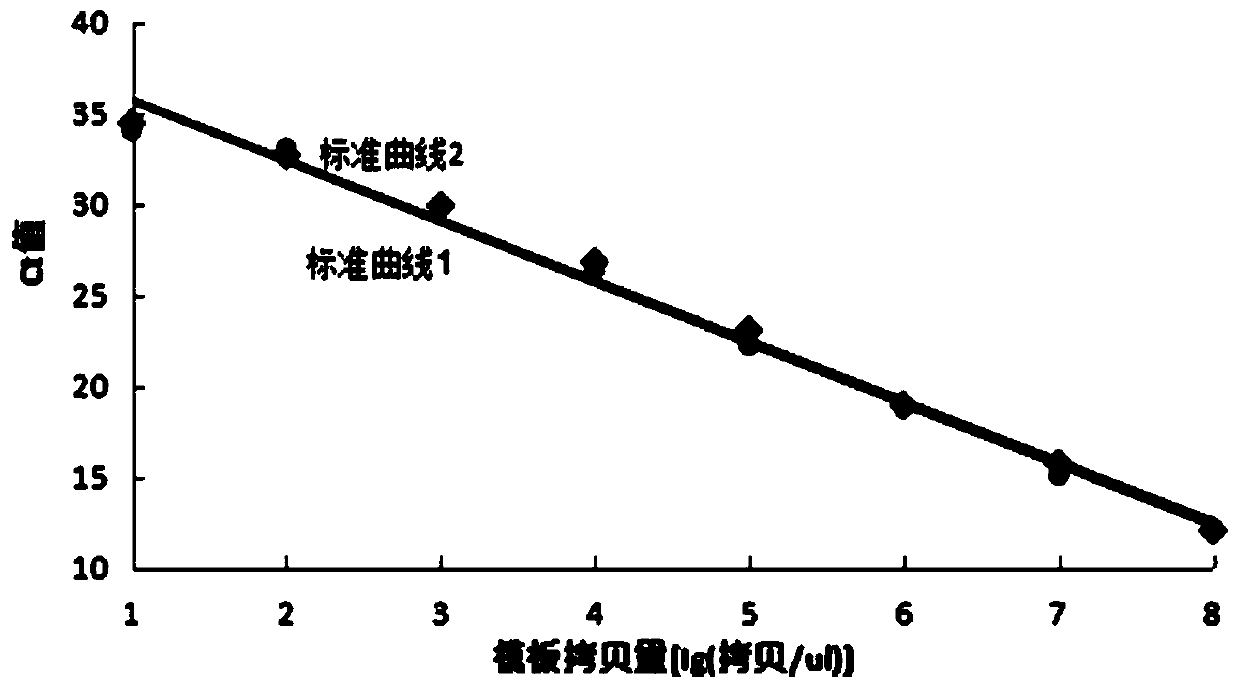
[0097] Use specific primers (SEQ ID No.2 2063MU, SEQ ID No.3 2064MU) and non-specific primers (SEQID No.1 23NU) to amplify the corresponding mutant standard respectively (do 3 holes for each reaction, Ct=MeanCt±SD ), with the logarithm value of the standard substance concentration as the abscissa, and the corresponding Ct value as the ordinate, the corresponding specificity and non-specificity standard curves are obtained, such as figure 1 and figure 2 As shown, the standard curves almost overlap, indicating that the introduction of non-matching base (I) in the specific primer sequence does not affect the amplification efficiency of the primers, the template amount has a good correlation with the Ct value, and the template can be quantified. in, figure 1 Middle standard curve 1: The straight line is the specific standard curve obtained by amplifying the A2063G ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com