Chimeric protein pAgoE, construction method and applications thereof, chimeric protein pAgoE using guide, and construction method and applications thereof
A chimeric protein and construction method technology, applied in chemical instruments and methods, biochemical equipment and methods, hybrid peptides, etc., can solve problems such as unclear methods and reproducibility crises
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0173] Example 1: Construction and genome editing of the chimeric protein Fok-RsAgo.
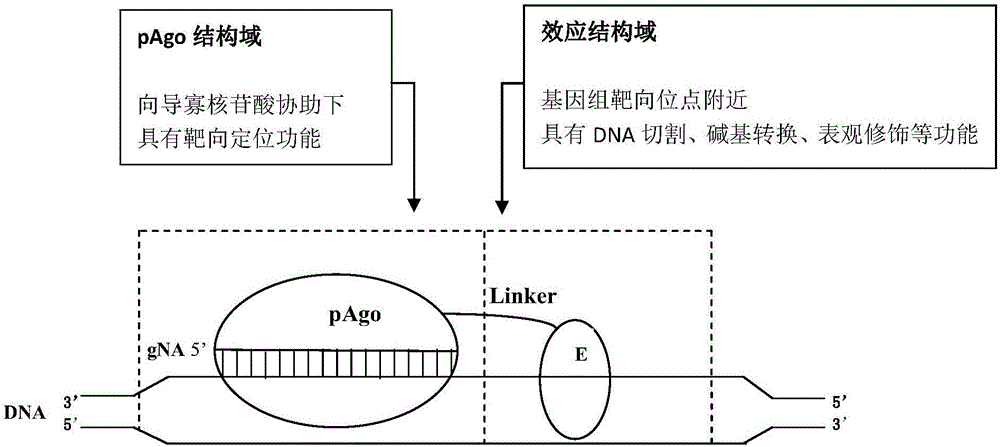
[0174] From the 319 candidate pAgos in Table 3, RsAgo was selected as the pAgo domain; FokI in the endonuclease was selected as the E domain to construct the N-pAgo type pAgoE (hereinafter referred to as Fok-RsAgo), and the Fok-RsAgo was used to carry the corresponding Combine the plasmids of gNA and HR fragments, transform yeast and subculture, and detect gene editing.
[0175] 1. Construction and expression of Fok-RsAgo expression plasmid:
[0176]According to the amino acid sequence of the RsAgo protein (NCBI accession number: ABP72561.1) of Rhodobacter sphaeroides ATCC 17025, such as SEQ ID NO: 1, according to the base preference of yeast, the rsago gene sequence encoding the RsAgo protein was designed, and the overlapping extension PCR was used technology, artificially synthesized and T-cloned the gene sequence, and obtained rsago‐pEASY. According to the amino acid sequence of the end...
Embodiment 2
[0185] Example 2: Construction and genome editing of the chimeric protein Fok-MpAgo.
[0186] From the 319 candidate pAgos in Table 3, MpAgo was selected as the pAgo domain; FokI in the endonuclease was selected as the E domain to construct the N‐pAgo type pAgoE (hereinafter referred to as Fok‐MpAgo), and the Fok‐MpAgo was used to carry the corresponding The plasmids of gNA and HR fragments are combined to transform yeast and edit its genome.
[0187] 1. Construction and expression of Fok-MpAgo expression plasmid:
[0188] According to the amino acid sequence of the MpAgo protein of Marinitoga piezophila (NCBI accession number: WP_014295921.1), such as SEQ ID NO: 2, according to the base preference of yeast, the mpago gene sequence encoding the MpAgo protein was designed, and the overlapping extension PCR technique was used to The gene sequence was artificially synthesized and T-cloned to obtain mpago-pEASY. Using the foki-pEASY obtained in step 1 of Example 1, select GGGGS ...
Embodiment 3
[0195] Example 3: Construction and genome editing of the chimeric protein Fok-dMpAgo.
[0196] From the 319 candidate pAgos in Table 3, the PIWI inactivated mutant dMpAgo of MpAgo was selected as the pAgo domain, and the FokI in the endonuclease was selected as the E domain to construct the N‐pAgo type pAgoE (hereinafter referred to as Fok‐dMpAgo). Fok‐dMpAgo cooperates with plasmids carrying corresponding gNA and HR fragments to transform yeast and edit its genome.
[0197] 1. Construction and expression of Fok‐dMpAgo expression plasmid:
[0198] According to the amino acid sequence of the MpAgo protein of Marinitoga piezophila (NCBI accession number: WP_014295921.1), such as SEQ ID NO: 2, according to the base preference of yeast, the mpago gene sequence encoding the MpAgo protein was designed, and the overlapping extension PCR technique was used to The gene sequence was artificially synthesized and T-cloned to obtain mpago-pEASY. Using the obtained mpago-pEASY plasmid as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com