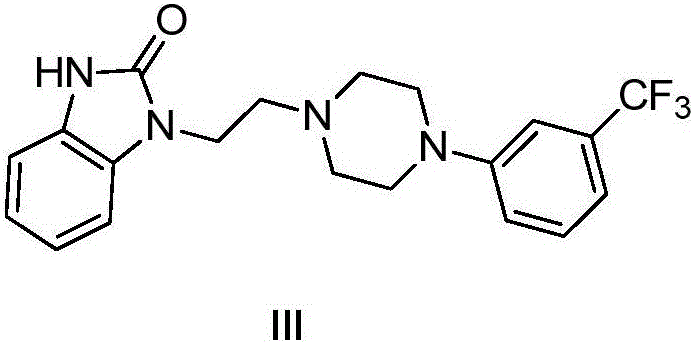
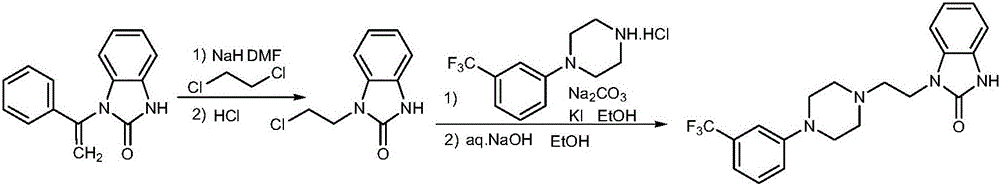
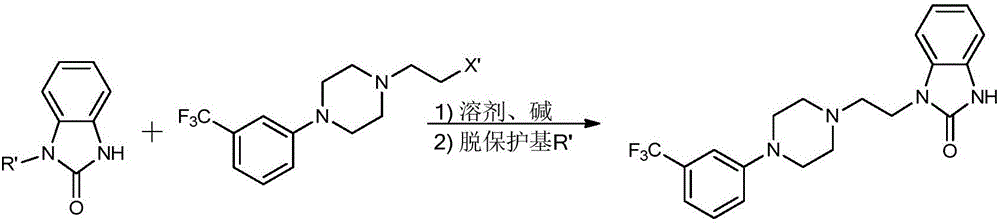
Benzimidazole compound and preparation method and application thereof
A technology of benzimidazoles and compounds, applied in the field of medicinal chemistry, can solve problems such as low yield, high preparation cost, and difficult removal of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Example 1 2-Ethoxy-1H-benzimidazole
[0114]
[0115] Add o-phenylenediamine (20g, 185mmol) and tetraethyl orthoformate (37.3g, 194mmol) into acetic acid (11.1g, 185mmol) and heat at 80°C for 1.5h. TLC showed the reaction was complete. Concentrate the reaction solution to a small volume, then pour it into 200ml of water, stir for 10 minutes, filter with suction, wash the filter cake once with water, and dry at 65°C to obtain 27g of the product with a yield of 90%.
[0116] 1 H NMR(400MHz,DMSO)δ11.78(s,1H),7.35(s,1H),7.21(s,1H),7.07–6.96(m,2H),4.47(q,J=7.1Hz,2H) ,1.37(t,J=7.1Hz,3H).
[0117] ESI-[M+H] + =162.99
Embodiment 2
[0118] Example 2 2-Ethoxy-1H-benzimidazole
[0119]
[0120] Add o-phenylenediamine (0.8kg, 7.39mol) and tetraethyl orthoformate (1.49kg, 7.76mol) into acetic acid (444g) and react at 70°C for 2h. TLC showed the reaction was complete. The reaction solution was cooled to room temperature, and 5% potassium hydroxide aqueous solution (6.4 L) was slowly added, and the reaction system was stirred for 1 h. The precipitated solid was filtered, the filter cake was washed with water, and dried to obtain the target compound as a white solid (1.13kg, 94%). 1 H NMR (400MHz, DMSO-d 6 )δ11.80(brs,1H),7.29(m,2H),7.03(m,2H),4.48(q,J=7.1Hz,2H),1.37(t,J=7.1Hz,3H).ESI- MS(m / z):163.5[M+H] + ,161.2[M-H] - ; HPLC: the retention time is 12.2min, and the purity is 96.3%.
Embodiment 3
[0121] Example 3 1-(2-chloroethyl)-2-ethoxy-1H-benzimidazole
[0122]
[0123] The product of Example 1 (2g, 12.3mmol), potassium carbonate (6.8g, 49.4mmol), 1,2-dichloroethane (6.1g, 61.5mmol) was added to acetonitrile (10ml) and refluxed for 15h, TLC showed a reaction completely. After suction filtration, the filter cake was washed with ethyl acetate, and the filtrate was concentrated to dryness to obtain 2.77 g, and the crude product yield was 99.8%.
[0124] 1H NMR (400MHz, DMSO) δ7.46–7.38(m,2H),7.14–7.05(m,2H),4.55(q,J=7.1Hz,2H),4.37(t,J=5.9Hz,2H) ,3.96(t,J=5.9Hz,2H),1.41(t,J=7.1Hz,3H).
[0125] ESI-[M+H] + =225.04
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com