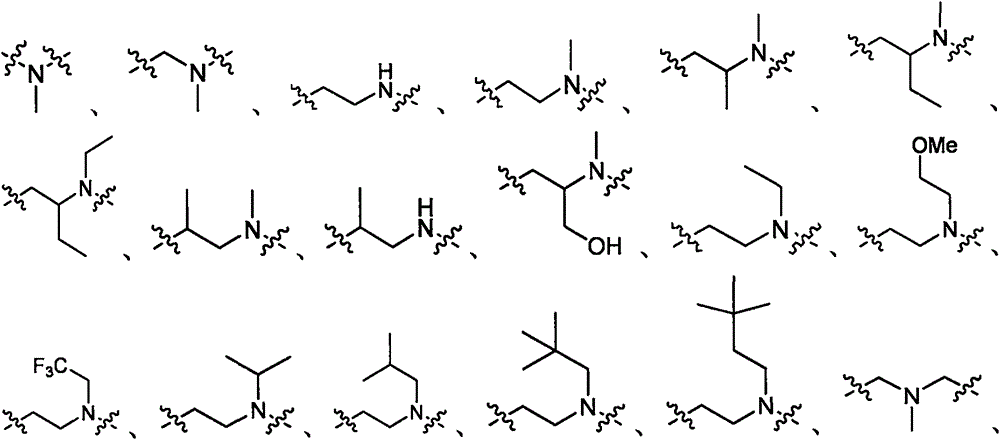
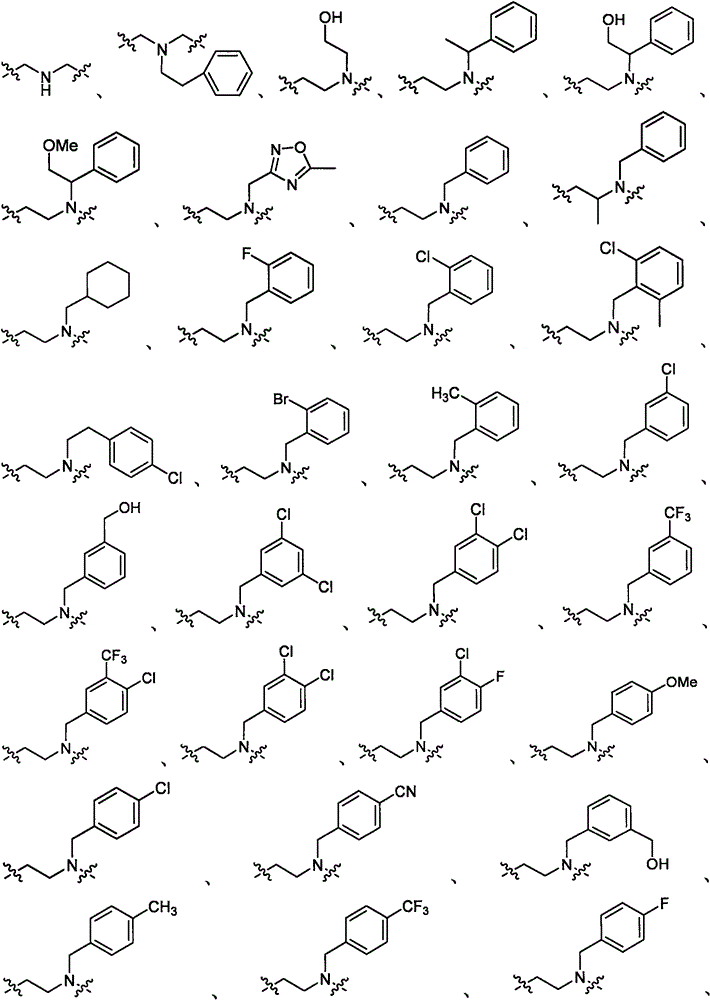
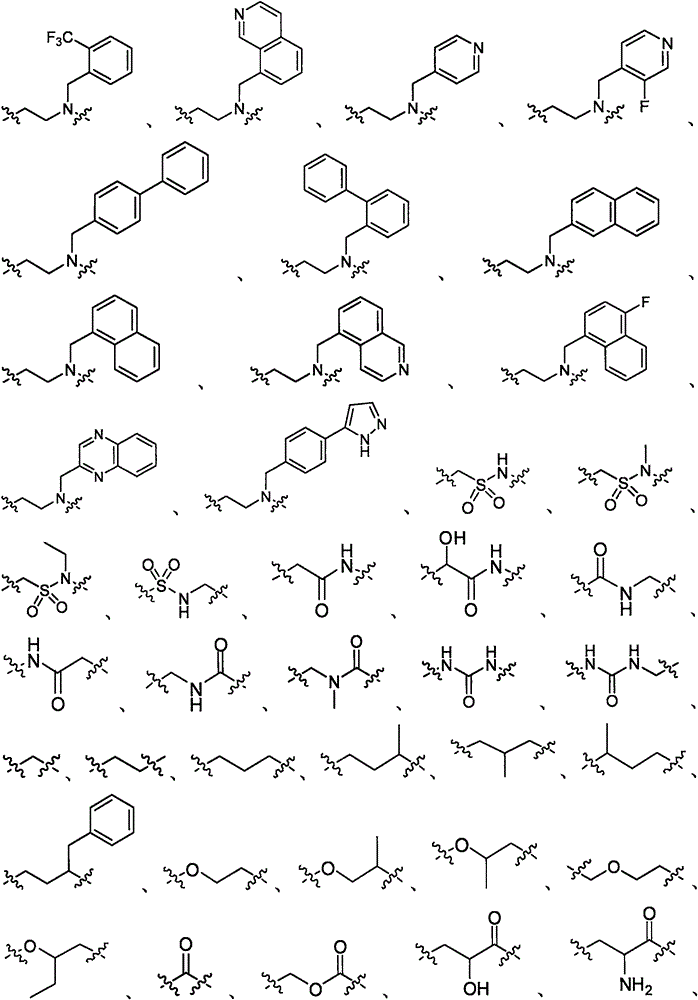
Substituted amino triazoles, and methods using same
一种卤代烷基、卤代烷氧基的技术,应用在取代的氨基三唑及其使用领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0619] Scheme 1 illustrates the preparation of aminotriazolopiperazines. The reaction produces three possible tautomers, which are interchangeable. For convenience, only one triazole tautomer is depicted throughout the specification. In one method (Method A), substituted piperazines and dimethyl cyanocarbonimidodithioate were combined in anhydrous acetonitrile and refluxed overnight. After the intermediate was formed, hydrazine hydrate monohydrate was added to the reaction mixture, and reflux was continued until the reaction was complete. In another method (Method B), the above reaction was carried out by microwave irradiation at 160° C. for 1 hour for each step.
[0620] Scenario 2:
[0621]
[0622] Similar chemistry to BOC-protected piperazines can be used to prepare unsubstituted aminotriazolopiperazines as intermediates for further synthesis, eg, by reductive amination (Scheme 2). Other alkylation, acylation, or sulfonylation reactions may also be used to add subst...
Embodiment 1
[0636] Example 1: 5-(4-(2-(4-fluorophenoxy)ethyl)piperazin-1-yl)-1H-1,2,4-triazol-3-amine.
[0637]
[0638] Step 1: Methyl N-cyano-4-(2-(4-fluorophenoxy)ethyl)piperazine-1-carbimidothioate
[0639]
[0640] To a 100 mL single port RBF equipped with nitrogen inlet, reflux condenser, and bleach trap was added 1-[2-(4-fluorophenoxy)ethyl]piperazine (0.0553 g, 0.2466 mmol) , dimethyl cyanodithioimidate (0.0361 g, 0.2466 mmol), and anhydrous acetonitrile (20 mL). The reaction solution was refluxed overnight under nitrogen. TLC and MS confirmed the presence of the desired intermediate. The reaction solution was carried forward without purification. C 15 h 19 FN 4 Calculated ESI-LCMS m / z for OS: predicted 322.4; found 323.2 [M+H] + .
[0641] Step 2: 5-(4-(2-(4-fluorophenoxy)ethyl)piperazin-1-yl)-1H-1,2,4-triazol-3-amine
[0642] To the reaction solution from step 1 was added hydrazine hydrate monohydrate (0.1929 g, 2.466 mmol, 187 μL). The solution was refluxed for 16...
Embodiment 2
[0643] Example 2: 5-(4-(2-(4-Chlorophenoxy)ethyl)piperazin-1-yl)-1H-1,2,4-triazol-3-amine.
[0644]
[0645] Step 1: Methyl 4-(2-(4-chlorophenoxy)ethyl)-N-cyanopiperazine-1-thioimidate
[0646]
[0647] Prepared in a similar manner to Example 1 (Step 1) from 1-[2-(4-chlorophenoxy)-ethyl]piperazine. C 15 h 19 ClN 4 Calculated ESI-LCMS m / z for OS: predicted 338.9; found 339.2 [M+H] + .
[0648] Step 2: 5-(4-(2-(4-Chlorophenoxy)ethyl)piperazin-1-yl)-1H-1,2,4-triazol-3-amine
[0649] Prepared and purified in a similar manner to Example 1 (step 2) from methyl 4-(2-(4-chlorophenoxy)ethyl)-N-cyanopiperazine-1-thioimidate, The desired product was obtained as a white solid. (0.100 g, 62% yield). 1 H NMR (CD 3 OD, 300MHz) δ (ppm) 7.09-6.99 (m, 4H), 5.48 (s, 2H), 4.38 (t, J = 5.0Hz, 2H), 3.67 (t, J = 5.0Hz, 6H), 3.35 ( s, 2H); C 14 h 19 ClN 6 Calculated ESI-LCMS m / z for O: predicted 322.8; found 323.2 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com