Synthesis method of photoelectric material intermediate 9-(4'-chlorobiphenyl-2-yl) carbazole
A synthesis method and technology of chlorinated biphenyls, applied in the direction of organic chemistry, etc., to achieve the effects of facilitating industrial production, simplifying post-processing steps, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
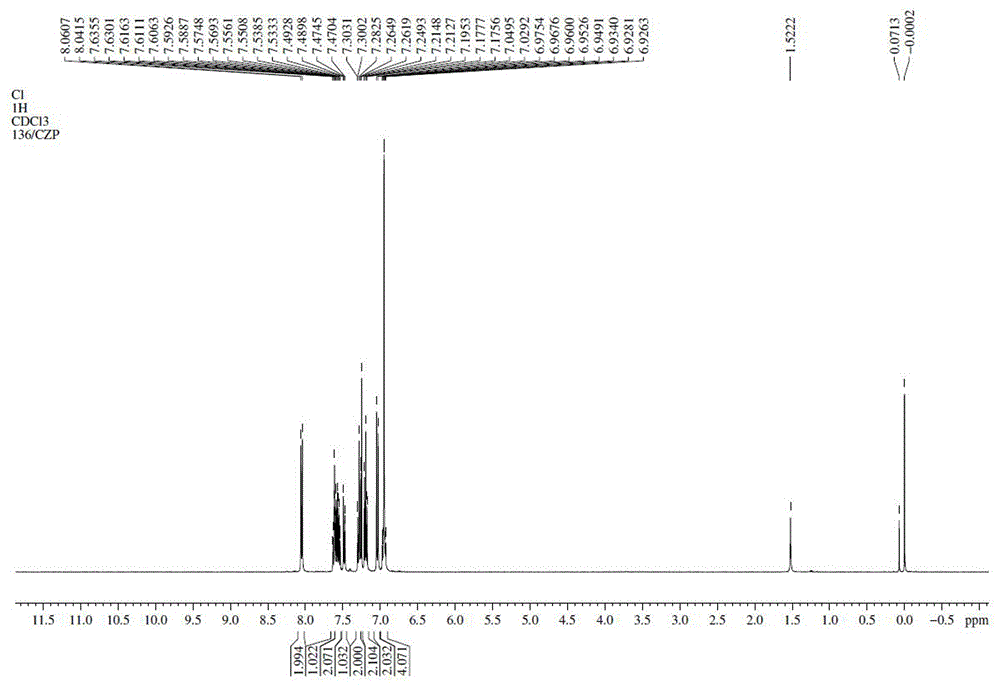
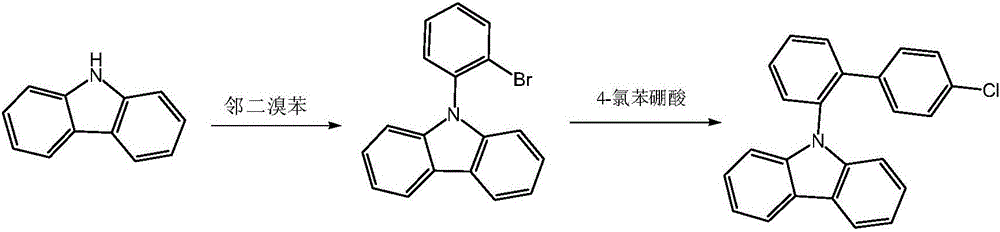
[0015] (1) Put 8.36g (0.05mol) of carbazole, 17.69g (0.075mol) of o-dibromobenzene, and 17.97g (0.13mol) of potassium carbonate in the reactor, stir, replace with nitrogen for 30min, and add cuprous iodide 1.91g, L-lysine 1.46g, heated to 163°C in the system, after 38h, TLC detected no raw materials, added a large amount of water to terminate the reaction, stirred and precipitated solid, filtered and dried to obtain solid 9-(2-bromophenyl ) carbazole 13.37g, yield 83%.
[0016] (2) 9.67g (0.03mol) of 9-(2-bromophenyl) carbazole, 5.01g (0.032mol) of p-chlorophenylboronic acid, 11.20g (0.081mol) of potassium carbonate, and 100mL of solvent (toluene: EtOH: H 2 O=10:2:1) placed in the reactor, stirred, replaced with nitrogen for 30min, added Pd 2 (dba) 3 0.055g, X-phos 0.029g, heat to reflux, after 4h, TLC detects that there is no raw material, add water to terminate the reaction, separate the water layer, filter the organic layer through silica gel adsorption, remove the solve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com